��Ŀ����
��8�֣�
�Ȼ����һ����Ҫ�Ļ���ԭ�ϣ�Ӧ�ù㷺��
��1��ʵ����ͨ����NH4Cl������Ca(OH)2�����Ϲ�����ȡ������
д��ʵ������ȡ�����ķ�Ӧ����ʽ ������
��2����Ũ�Ȼ����Һ��������̨Ļ�����Ż���ԭ���� ������ĸ����
��Ļ�����Ż������ ��Ļ������������ ���Ȼ�立ֽ������������������¶�
���Ȼ�立ֽ��������������˲��ֿ���
��3��ʵ���ҿ���NH4Cl��Һ�뱥�͵�NaNO2��Һ��Ӧ��ȡ�����ĵ�������Ӧ����ʽΪ��
NaNO2��NH4Cl NaCl��N2����2H2Oʵ��װ����ͼ��ʾ���Իش�
NaCl��N2����2H2Oʵ��װ����ͼ��ʾ���Իش�
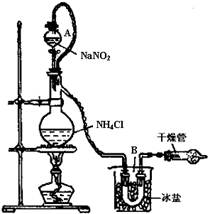
��װ���У����ֵķ�Һ©����������ƿ֮�����ӵĵ�������������� ����д��ţ���
a����ֹ������Һ����
b����֤ʵ��װ�ò�©��
c��ʹ����NaNO2��Һ������
�ڼ���ǰ������е�һ������������ ���� ��
�Ȼ����һ����Ҫ�Ļ���ԭ�ϣ�Ӧ�ù㷺��
��1��ʵ����ͨ����NH4Cl������Ca(OH)2�����Ϲ�����ȡ������
д��ʵ������ȡ�����ķ�Ӧ����ʽ ������
��2����Ũ�Ȼ����Һ��������̨Ļ�����Ż���ԭ���� ������ĸ����
��Ļ�����Ż������ ��Ļ������������ ���Ȼ�立ֽ������������������¶�
���Ȼ�立ֽ��������������˲��ֿ���
| A���٢� | B���ۢ� | C���٢� | D���ڢ� |
NaNO2��NH4Cl
 NaCl��N2����2H2Oʵ��װ����ͼ��ʾ���Իش�
NaCl��N2����2H2Oʵ��װ����ͼ��ʾ���Իش�
��װ���У����ֵķ�Һ©����������ƿ֮�����ӵĵ�������������� ����д��ţ���
a����ֹ������Һ����
b����֤ʵ��װ�ò�©��
c��ʹ����NaNO2��Һ������
�ڼ���ǰ������е�һ������������ ���� ��
��1��2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��2��B
��3��c�����������
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O����2��B
��3��c�����������
�����������ȡ
��1��ʵ����ͨ������μ�������ȡ������2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��2������ȼ�յıر�������
ͨ����ӦNH4Cl NH3��+H2O�ֽ����������������¶ȣ�ʹĻ���ﲻ���Ż�㣻���߷ֽ�����İ����ɸ���������ʹ�䲻��ȼ�գ���ѡB
NH3��+H2O�ֽ����������������¶ȣ�ʹĻ���ﲻ���Ż�㣻���߷ֽ�����İ����ɸ���������ʹ�䲻��ȼ�գ���ѡB
��3���ٷ�Һ©����������ƿ֮�����ӵĵ��ܿ�ƽ������֮���ѹǿ���÷�ӦҺ���ڵ���
�ڶ�����ȡ�����ʵ��װ�ã������Լ��ز�����
��1��ʵ����ͨ������μ�������ȡ������2NH4Cl+Ca(OH)2
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O����2������ȼ�յıر�������
ͨ����ӦNH4Cl
 NH3��+H2O�ֽ����������������¶ȣ�ʹĻ���ﲻ���Ż�㣻���߷ֽ�����İ����ɸ���������ʹ�䲻��ȼ�գ���ѡB
NH3��+H2O�ֽ����������������¶ȣ�ʹĻ���ﲻ���Ż�㣻���߷ֽ�����İ����ɸ���������ʹ�䲻��ȼ�գ���ѡB��3���ٷ�Һ©����������ƿ֮�����ӵĵ��ܿ�ƽ������֮���ѹǿ���÷�ӦҺ���ڵ���
�ڶ�����ȡ�����ʵ��װ�ã������Լ��ز�����

��ϰ��ϵ�д�
�����Ŀ
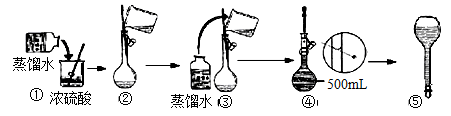
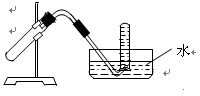

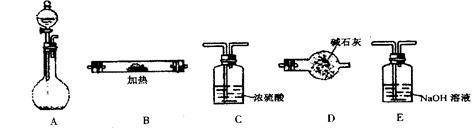
 ������ ��
������ �� ����˳���ǣ�����ĸ��������������
����˳���ǣ�����ĸ�������������� ������������E
������������E