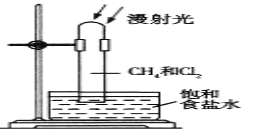
��Ŀ����
����Ŀ������A��B��C��D��E����Ԫ�أ����Ƕ�����Ԫ�أ�����A��B��Cԭ���������ε����������1��B�������ӵĵ��Ӳ�ṹ��Neԭ����ͬ��2gB����������100ml 0.5mol/L��H2SO4ǡ����ȫ��Ӧ��B������D���ʷ�Ӧ����γ����ӻ�����BD2��B������E���ʿ��γɻ�����BE��D�������ӱ�B�������Ӷ�һ�����Ӳ㣬��E��������B�������ӵ��Ӳ�ṹ��ͬ��
��1��BԪ�ص�����________��
��2���γɻ�����A2E2�Ļ�ѧ�������ǣ�____________________________��
��3���õ���ʽ��ʾ������BD2���γɹ��̡�____________________________��
��4��A�����������ˮ��Һ��C���ʷ�����Ӧ�����ӷ���ʽ��___________��
���𰸡�þԪ�����Ӽ����Ǽ��Լ�![]() 2OH-+2Al+2H2O=2AlO2-+3H2��
2OH-+2Al+2H2O=2AlO2-+3H2��
��������
A��B��C��D��E���ֶ�����Ԫ����B������D���ʷ�Ӧ����γ����ӻ�����BD2������֪BΪ+2�ۡ�DΪ-1����B�������ӵĵ��Ӳ�ṹ��Neԭ����ͬ����BΪMg��2g MgO��100ml 0.5mol/L��H2SO4ǡ����ȫ��Ӧ��A��B��Cԭ���������ε����������1������֪AΪNa��CΪAl��D�������ӱ�B�������Ӷ�һ�����Ӳ�����DΪCl��B������E���ʿ��γɻ�����BE��EΪ-2������E��������B�������ӵ��Ӳ�ṹ��ͬ����EΪOԪ����Ȼ������Ԫ�ؼ��䵥�ʡ�������������������
A��B��C��D��E���ֶ�����Ԫ�أ�B������D���ʷ�Ӧ����γ����ӻ�����BD2������֪BΪ+2�ۡ�DΪ-1�ۣ�B�������ӵĵ��Ӳ�ṹ��Neԭ����ͬ����BΪMg��2g MgO��100ml 0.5mol/L��H2SO4ǡ����ȫ��Ӧ��A��B��Cԭ���������ε����������1������֪AΪNa��CΪAl��D�������ӱ�B�������Ӷ�һ�����Ӳ㣬��DΪCl��B������E���ʿ��γɻ�����BE��EΪ-2�ۣ���E��������B�������ӵ��Ӳ�ṹ��ͬ����EΪOԪ�أ�
(1)��������������֪����BΪþ��
��ˣ�������ȷ������þ��
(2)������Na2O2�������Ӽ������ۼ���
��ˣ�������ȷ���������Ӽ������ۼ���
(3)MgCl2����������þ���ӹ�������Mgԭ�ӡ�Clԭ�ӵ���ʽ��ʾ���γɹ���Ϊ��![]() ��
��
��ˣ�������ȷ������![]() ��
��
(4)Al���������Ʒ�Ӧ��Ӧ����ƫ�����������������ӷ���ʽΪ��2Al +2OH-+2H2O=2AlO2-+3H2����
��ˣ�������ȷ������2Al +2OH-+2H2O=2AlO2-+3H2����

����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol��L-1HCl����Һ�����к͵ζ����ü�����ָʾ������
��ش��������⣺
��1���ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ ��ʢװ���������������Ϊ ���ζ����յ����ɫ�仯Ϊ ��
��2������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ ��
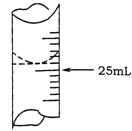
��3����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.29 |
2 | 25.00 | 1.56 | 31.30 |
3 | 25.00 | 1.00 | 27.31 |
ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ ��������λ��Ч���֣���
��4��������Щ������ʹ�ⶨ���ƫ�� ������ţ���
A.��ƿ������ˮϴ�������ô���Һ��ϴ
B.��ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C.�ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D.�ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
����Ŀ��ʵ��С���Ʊ�������أ�K2FeO4����̽�������ʡ�
���ϣ�K2FeO4Ϊ��ɫ���壬����KOH��Һ�������Ի�������Һ�п��ٲ���O2���ڼ�����Һ�н��ȶ���
��1��K2FeO4��Ϊ��Ч�����ˮ��������ԭ����______________________________��
��2���Ʊ�K2FeO4���г�װ���ԣ�

��AΪ��������װ�á�A�з�Ӧ����ʽ��_____________________________��
�ڸ�װ�������Բ�������ƣ���θĽ���____________________________��
�۸Ľ���B�еõ���ɫ�������Һ��B��Cl2�����ķ�Ӧ��3Cl2+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O�������____________________________��
��3��̽��K2FeO4�����ʣ��Ľ����ʵ�飩
��ȡB����ɫ��Һ������ϡ���ᣬ��������ɫ���壬����Һa�������������к���Cl2��Ϊ֤���Ƿ�K2FeO4������Cl��������Cl2��������·�����
������ | ȡ����a���μ�KSCN��Һ����������Һ�ʺ�ɫ�� |
������ | ��KOH��Һ���ϴ��B�����ù��壬����KOH��Һ��K2FeO4�ܳ����õ���ɫ��Һb��ȡ����b���μ����ᣬ��Cl2������ |
a���ɷ���������Һ����֪a�к���______���ӣ��������ӵIJ��������ж�һ����K2FeO4��Cl����������������_________________________�������÷���ʽ��ʾ����
b���������֤��K2FeO4������Cl������KOH��Һϴ�ӵ�Ŀ����______________��
�ڸ���K2FeO4���Ʊ�ʵ��ͷ�����ʵ�����Cl2��![]() ��������ǿ����ϵ�෴��ԭ����_____________��
��������ǿ����ϵ�෴��ԭ����_____________��
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã�����ѧ�����ش��������⣺
�� ���� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
��1�����ԭ�ӽṹʾ��ͼΪ_________��
��2���ڵ���̬�⻯����ӵĽṹʽΪ_____________��
��3���ڡ��۵���ۺ������������ǿ������˳����________________�����ѧʽ��
��4���ݡ���Ԫ�صĽ�����ǿ������Ϊ___________�������������С�����䡱��
��5���ܡ��ݡ����γɵļ����Ӱ뾶����_______�������������С�����䡱��
��6���١��ܡ���Ԫ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д�����ĵ���ʽ��_______��