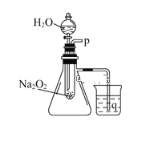
��Ŀ����
����Ŀ����ʹ������к͵ζ����ⶨ���۰״�������(g/100 mL)��
��.ʵ�鲽�裺
��1����ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100 mL����ƿ�ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00 mL����ƿ�У������еμ�2��____��ָʾ����
��3����ȡʢװ0.100 0 mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________ mL��
![]()
��4���ζ�����___________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
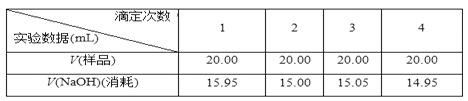
��.ʵ���¼��
��.ʵ�鴦����
��5��ijͬѧ�ڴ�������ʱ����ã�ƽ�����ĵ�NaOH��Һ�����V��(15.95��15.00��15.05��14.95)/4 mL��15.24 mL�� ָ�����ļ���IJ�����֮����_____������ȷ���ݴ������ɵ����۰״���������______ g/100 mL��(���������λ��Ч����)
��6���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����(��д���)________��
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ b����ʽ�ζ��ܼ���ζ�ǰ�����ݣ��ζ�����ʧ
c����ƿ�м������״���Һ���ټ�����ˮ d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
���𰸡� ��̪ 0.70 ���һ�ε���ʱ����Һ����ɫ��Ϊ��ɫ��������ڲ��ٸı� ��һ��ʵ���������̫������ 4.5 ab
����������.��2����ǿ��ζ������������÷�̪��Һ��ָʾ������ȷ�𰸣���̪��
��3������ͼʾ����֪ÿһ����λ�ַ�Ϊ10��С��λ��ÿһ��С��λΪ0.10 mL��Һ�尼Һ��Ķ���Ϊ0.70 mL����ȷ����0.70��
��4������Һ����ɫͻȻ��Ϊ��ɫ���Ұ��������ɫ���ֲ������ﵽ�ζ��յ�����ȷ�𰸣����һ�ε���ʱ����Һ����ɫ��Ϊ��ɫ��������ڲ��ٸı䡣
��.��5����һ��ʵ���������̫������������ƽ�����ĵ�NaOH��Һ�����V��(15.00��15.05��14.95)/3��15.00 mL��������������ư�1:1�����кͷ�Ӧ������n(NaOH)=n(CH3COOH)= 15.00��10-3��0.1=1.5��10-3 mol��20.00 mL��Һ����n(CH3COOH)= 1.5��10-3 mol��100mL��Һ�к���n(CH3COOH)= 7.5��10-3 mol����ɵ����۰״���������7.5��10-3 ��60 g��10=4.5 g/100 mL����ȷ������һ��ʵ���������̫������������4.5��
��. ��ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ����ɱ�ҺŨ�ȱ�С���������ƫ�����ⶨ���ƫ����a��ȷ����ʽ�ζ��ܼ���ζ�ǰ�����ݣ��ζ�����ʧ���������������Һ�Ķ���ƫ�����ⶨ���ƫ����b��ȷ����ƿ�м������״���Һ���ټ�����ˮ����Ӱ�����ʵ������ⶨ�����Ӱ����c��������ƿ�ڵζ�ʱ����ҡ����������Һ�彦������ɴ���Һ���ʵ�����С���ⶨ���ƫ����d��������ȷѡ����ab��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ����ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ���䷴Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2==3Na2S2O3+CO2���÷�Ӧ��H>0����ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O���������¡�

��1������װ����ͼ��ʾ��

��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ��� ____________ ������SO2����Ч�ʵ͵�ʵ��������B����Һ _________________��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ�� __________________ ������һ����
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH��10.2����
��� | ʵ����� | Ԥ������ | ���� |
�� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬ _________�� | �а�ɫ�������� | ��Ʒ��NaCl |
�� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬________�� | �а�ɫ�������ɣ��ϲ���ҺpH>10.2 | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡa g KIO3����Է�������Ϊ214�����������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
�������� ��Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����ΪV mL��
��c(Na2S2O3)��_________mol��L��1��
��֪��IO3-+5I-+6H+= 3I2��3H2O ��2S2O32����I2=S4O62����2I��
��4��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�Na2S2O3Ũ�ȿ���__________����������Ӱ��������ƫ��������ƫ��������ԭ����_________________________________���������ӷ���ʽ��ʾ����