��Ŀ����
��1�������£���0.1mol?L-1������Һ��0.06mol?L-1����������Һ�������Ϻû����Һ��pH=
��2�������£�pH=a��10�����ijǿ����pH=b��1�����ijǿ���Ϻ���Һ�����ԣ���a��b����Ĺ�ϵ
��3��ij�¶�ʱ��ˮ�����ӻ�KW=1��10-13������¶�
��4�������£�pH=8��NH4Cl��Һ�У�c��Cl-��-c��NH4+��=
12
12
����Ϻ���Һ����ı仯���Բ��ƣ���2�������£�pH=a��10�����ijǿ����pH=b��1�����ijǿ���Ϻ���Һ�����ԣ���a��b����Ĺ�ϵ
a+b=15
a+b=15
����Ϻ���Һ����ı仯���Բ��ƣ���3��ij�¶�ʱ��ˮ�����ӻ�KW=1��10-13������¶�
��
��
25�棨ѡ���������������=�������������¶���pH=2��ϡ����aL��pH=12������������ҺbL��ϣ������û��Һ��pH=11����a��b=9��11
9��11
����Ϻ���Һ����ı仯���Բ��ƣ���4�������£�pH=8��NH4Cl��Һ�У�c��Cl-��-c��NH4+��=
9.9��10-7mol/L
9.9��10-7mol/L
����������1��0.06mol?L-1����������Һc��OH-��=0.12mol/L�����ߵ���������Һ�ʼ��ԣ��ȼ�������Һ��c��OH-�����ٽ�����ӻ�������������Һ��c��H+�����Ӷ�ȷ�������Һ��pH��
��2��ǿ���ǿ������Һ�����ԣ�˵������c��H+�����ڼ���c��OH-����
��3��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬ˮ�����ӻ����������ݻ����Һ��c��OH-��=
���㣻
��4�����ݵ���غ����c��Cl-��-c��NH4+����
��2��ǿ���ǿ������Һ�����ԣ�˵������c��H+�����ڼ���c��OH-����
��3��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬ˮ�����ӻ����������ݻ����Һ��c��OH-��=
| n(OH-)-n(H+) |
| V(��)+V(��) |
��4�����ݵ���غ����c��Cl-��-c��NH4+����
����⣺��1������ͼ���������1L��0.06mol?L-1����������Һc��OH-��=0.12mol/L��0.1mol?L-1������Һ��������Ũ����0.1mol/L��
���ߵ���������Һ�ʼ��ԣ������Һ��c��OH-��=
mol/L=0.01mol/L�������Һ��������Ũ��Ϊ10-12 mol/L�����Ի����Һ��pH=12��
�ʴ�Ϊ��12��
��2��ǿ���ǿ������Һ�����ԣ�˵������c��H+�����ڼ���c��OH-��������10��10-a=1��10b-14������a+b=15��
�ʴ�Ϊ��a+b=15��
��3��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬ˮ�����ӻ���������ij�¶�ʱ��ˮ�����ӻ�KW=1��10-13����10-14����������¶ȣ�25�棻�����Һ��������Ũ����10-11 mol/L��c��OH-��=10-3 mol/L��
�����Һ��c��OH-��=
=
=10-3 mol/L��a��b=9��11��
�ʴ�Ϊ��9��11��
��4�����ݵ���غ����c��Cl-��-c��NH4+��=c��H+��-c��OH-��=10-6mol/L-10-8 mol/L=9.9��10-7mol/L��
�ʴ�Ϊ��9.9��10-7mol/L��
���ߵ���������Һ�ʼ��ԣ������Һ��c��OH-��=
| 0.12-0.1 |
| 2 |
�ʴ�Ϊ��12��
��2��ǿ���ǿ������Һ�����ԣ�˵������c��H+�����ڼ���c��OH-��������10��10-a=1��10b-14������a+b=15��
�ʴ�Ϊ��a+b=15��
��3��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬ˮ�����ӻ���������ij�¶�ʱ��ˮ�����ӻ�KW=1��10-13����10-14����������¶ȣ�25�棻�����Һ��������Ũ����10-11 mol/L��c��OH-��=10-3 mol/L��
�����Һ��c��OH-��=
| n(OH-)-n(H+) |
| V(��)+V(��) |
| 10-2mol/L��(b-a)L |
| (a+b)L |
�ʴ�Ϊ��9��11��
��4�����ݵ���غ����c��Cl-��-c��NH4+��=c��H+��-c��OH-��=10-6mol/L-10-8 mol/L=9.9��10-7mol/L��
�ʴ�Ϊ��9.9��10-7mol/L��
���������⿼����pH�ļ��㣬��ȷ������pH���㹫ʽ�ǽⱾ��ؼ����ѵ��ǣ�4���������Ӻ�笠�����Ũ�ȵIJ���ݵ���غ���������ɣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ

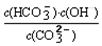 =2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH= ��
=2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH= ��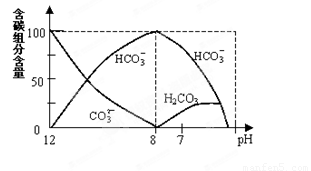
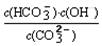 =2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH=
��
=2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH=
��