��Ŀ����
9��A��G�����л���������ǵ�ת����ϵ���£�
��ش��������⣺
��1����֪��6.0g������E��ȫȼ������8.8g C02��3.6g H20����ͬ������E������������������ܶ�Ϊ30����E�ķ���ʽΪ��C2H4O2��
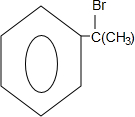
��2��AΪһȡ����������B�к���һ��������B����C�Ļ�ѧ����ʽΪ��
 ��
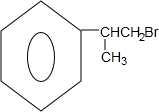
����3����B����D�ķ�Ӧ�������������ƴ���Һ�����ȣ�������Ӧ������ȡ����Ӧ����3����
��4��F���������������У���ṹ��ʽΪ
 ��F��NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽΪ��
��F��NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽΪ�� ��
����5����G��ͬ���칹���У�������λ�ϸ���һ��̼ԭ�ӣ��ұ����ϵ�һ��������ֻ��һ�ֵĹ���4�������к˴Ź�������������壬�ҷ������Ϊl��1����
 ����ṹ��ʽ����
����ṹ��ʽ����
���� E������������������ܶ�Ϊ30����Mr��E��=30��2=60��6.0gE�����ʵ�����0.1mol����ȫȼ�պ�����CO2�� H2O�����ʵ����ֱ�Ϊ$\frac{8.8g}{44g/mol}$=0.2mol��$\frac{3.6g}{18g/mol}$=0.2mol��������N��C��=$\frac{0.2mol}{0.1mol}$=2��N��H��=$\frac{0.2mol��2}{0.1mol}$=4����N��O��=$\frac{60-12��2-8}{16}$=2����E�ķ���ʽ��C2H4O2��AΪһȡ���������ɷ���ʽ��֪Ϊ����ͬϵ���AΪ ��A�������ڹ��������·���ȡ����Ӧ����B����B�к���һ��������BΪ
��A�������ڹ��������·���ȡ����Ӧ����B����B�к���һ��������BΪ ��B����ˮ�⣨ȡ������Ӧ����CΪ
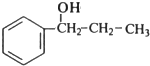
��B����ˮ�⣨ȡ������Ӧ����CΪ ��C��E����������ȡ������Ӧ����F�����F�ķ���ʽ��֪��EΪCH3COOH��FΪ
��C��E����������ȡ������Ӧ����F�����F�ķ���ʽ��֪��EΪCH3COOH��FΪ ��B��Cת�����õ�D��D���巢���ӳɷ�Ӧ����G����B��C��������ȥ��Ӧ����D����DΪ
��B��Cת�����õ�D��D���巢���ӳɷ�Ӧ����G����B��C��������ȥ��Ӧ����D����DΪ ����GΪ
����GΪ ���ݴ˽��
���ݴ˽��
��� �⣺E������������������ܶ�Ϊ30����Mr��E��=30��2=60��6.0gE�����ʵ�����0.1mol����ȫȼ�պ�����CO2�� H2O�����ʵ����ֱ�Ϊ$\frac{8.8g}{44g/mol}$=0.2mol��$\frac{3.6g}{18g/mol}$=0.2mol��������N��C��=$\frac{0.2mol}{0.1mol}$=2��N��H��=$\frac{0.2mol��2}{0.1mol}$=4����N��O��=$\frac{60-12��2-8}{16}$=2����E�ķ���ʽ��C2H4O2��AΪһȡ���������ɷ���ʽ��֪Ϊ����ͬϵ���AΪ ��A�������ڹ��������·���ȡ����Ӧ����B����B�к���һ��������BΪ
��A�������ڹ��������·���ȡ����Ӧ����B����B�к���һ��������BΪ ��B����ˮ�⣨ȡ������Ӧ����CΪ
��B����ˮ�⣨ȡ������Ӧ����CΪ ��C��E����������ȡ������Ӧ����F�����F�ķ���ʽ��֪��EΪCH3COOH��FΪ
��C��E����������ȡ������Ӧ����F�����F�ķ���ʽ��֪��EΪCH3COOH��FΪ ��B��Cת�����õ�D��D���巢���ӳɷ�Ӧ����G����B��C��������ȥ��Ӧ����D����DΪ
��B��Cת�����õ�D��D���巢���ӳɷ�Ӧ����G����B��C��������ȥ��Ӧ����D����DΪ ����GΪ
����GΪ ��
��
��1��������������֪��E�ķ���ʽΪC2H4O2���ʴ�Ϊ��C2H4O2��
��2����B����C�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3����B����D�� ������ȥ��Ӧ����
������ȥ��Ӧ���� ����Ӧ����Ϊ���������ƴ���Һ�����ȣ���������ķ�����֪��������Ӧ������ȡ����Ӧ����3����
����Ӧ����Ϊ���������ƴ���Һ�����ȣ���������ķ�����֪��������Ӧ������ȡ����Ӧ����3����
�ʴ�Ϊ���������ƴ���Һ�����ȣ�3��
��4��������������֪��F�Ľṹ��ʽΪ ��F��NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽΪ
��F��NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��5����G�� ����ͬ���칹���У�������λ�ϸ���һ��̼ԭ�ӣ��ұ����ϵ�һ��������ֻ��һ�ֵĽṹΪ
����ͬ���칹���У�������λ�ϸ���һ��̼ԭ�ӣ��ұ����ϵ�һ��������ֻ��һ�ֵĽṹΪ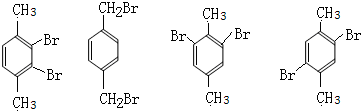 ����4�֣����к˴Ź�������������壬�ҷ������Ϊl��1�����ԶԳ��Ըߣ����Ժ���2����ͬ��ȡ�������Ҵ��ڶ�λ��Ϊ
����4�֣����к˴Ź�������������壬�ҷ������Ϊl��1�����ԶԳ��Ըߣ����Ժ���2����ͬ��ȡ�������Ҵ��ڶ�λ��Ϊ ��
��
�ʴ�Ϊ��4�� ��
��
���� ���⿼���л����ƶϣ��ؼ���ȷ��AΪ�ұ����ٽ��ת����ϵ������ʽ�ƶϣ���5����ͬ���칹����дΪ�״��㡢�ѵ㣬�Ѷ��еȣ�

| A�� | ��Ƭ��ϡ���ᷴӦ | B�� | ��������������Ȼ�立�Ӧ | ||
| C�� | ���ȵ�̼��CO2��Ӧ | D�� | ������������ȼ�� |
| ʵ �� | �� �� | ���� | |
| A | 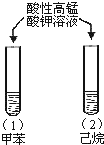 | �Թܣ�1������ɫ��ȥ�� �Թܣ�2������ɫδ�� | ����ʹ���Ļ�����ǿ |
| B | 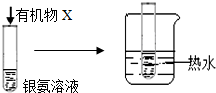 | �Թ��ڱ��� �������� | �л���X��һ������ȩ�� |
| C | �������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ����Һ������ | δ��ש��ɫ���� | ����δˮ�� |
| D | 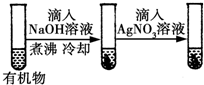 | ���һֻ�Թ�����dz��ɫ���� | �л����к�����ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ���� | Cl2 | Br2 | I2 | H2 | HF | HCl | HBr | HI |
| ���� | 243 | 193 | 151 | 436 | 568 | 432 | 366 | 298 |
��1���������ʱ������е�������ߵ���D ����A��B��C��D��
A��H2 B��Cl2 C��Br2 D��I2
��2�������⻯���У����ȶ�����A ����A��B��C��D���������ϱ��е����ݣ�������ȷ�ش�����������ܣ����ܡ�����ĸ�����Ԫ�طǽ�����Խǿ���γɵ���̬�⻯��Խ�ȶ���
A��HF B��HCl C��HBr D��HI
��3��X2+H2=2HX��X����Cl��Br��I���ķ�Ӧ�Ƿ��ȷ�Ӧ������ȡ����ȡ���
��4����ͬ�����£�X2��X����Cl��Br��I���ֱ���������Ӧ�������ĵ����ʵ���������ʱ���ų������յ�������������������д����ʽ����
| A | B | ||||||
| D | E | F | |||||
| C | G | H |
��2����һ�������£�A��E���γɻ�����Ļ�ѧʽ��NH3����ˮ��Һ�ʼ��ԣ���ᡱ��������С�����
��3��д��A��F�γɻ�����ĵ���ʽ
 ���û�������C���ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2����
���û�������C���ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2���� ��1����λʱ��������nmolO2��ͬʱ����2nmolNO2
��2����λʱ��������nmolO2��ͬʱ������2nmolNO
��3����NO2��NO��O2�����ʵ���Ũ�ȱ仯��ʾ��Ӧ���ʵı�Ϊ2��2��1��״̬
��4������������ɫ���ٸı��״̬��
| A�� | ��1����4�� | B�� | ��2����3�� | C�� | ��1����3����4�� | D�� | ��1����2����3����4�� |
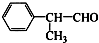 ����ҵ�ϳ�·�����£�
����ҵ�ϳ�·�����£�
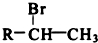
 ��R-����������
��R-���������� ����
����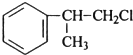 ��
��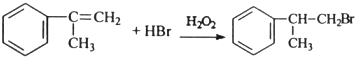 ��
�� +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2 +2H2O��
+2H2O��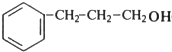 ��
�� ��
�� ��
��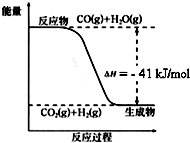 ��֪��ҵ�������ķ�ӦΪCO��g��+H2O��g��?CO2��g��+H2��g������Ӧ�����������仯��ͼ��ʾ����500��ʱ��ƽ�ⳣ�� K=9������2L���ܱ�������CO��ˮ��������ʼŨ�ȶ���0.1mol/L��10minʱ�ﵽƽ��״̬��
��֪��ҵ�������ķ�ӦΪCO��g��+H2O��g��?CO2��g��+H2��g������Ӧ�����������仯��ͼ��ʾ����500��ʱ��ƽ�ⳣ�� K=9������2L���ܱ�������CO��ˮ��������ʼŨ�ȶ���0.1mol/L��10minʱ�ﵽƽ��״̬��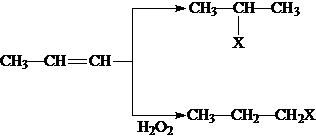 ��XΪ��ԭ�ӣ�
��XΪ��ԭ�ӣ� �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�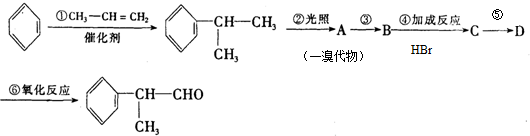
 ��
�� ��
�� ��
�� ����1�֣�
����1�֣�