��Ŀ����
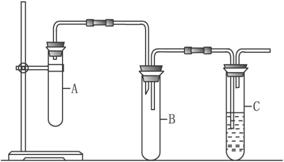
ij��ѧ����С��Ϊ̽��ͭ�����ᷴӦ��ԭ������ҪΪNOʱ�����Ũ�ȣ����ʵ��װ������ͼ��ʾ(���Ӻ���������������Ժֱ�װ�����Ӧ���Լ�)��
��.(1)ʵ�鿪ʼʱ�ȴ���K����ͨ��N2һ��ʱ�����K����ͭ˿(����)��������Ũ������д����ĺ���ɫ�������ɣ���ʱ��Ľ��У�������ɫ��dz����A�г�����ɫ����ʱ��
��װ��A�з�Ӧ�����ӷ���ʽΪ___________________________��
�ڽ�������ʵ�������___________________________________��
(2)��ʵ����װ��A���ŵ���____________________________��
��.��B��������Һϡ����200 mL����0.20 mol/L��NaOH��Һ���еζ���ʵ����������(����Ļӷ��ֽ⼰��Һ����ı仯���Բ���)��
ʵ���� | ����Һ���(mL) | NaOH��Һ���(mL) |
1 | 20.00 | 15.98 |
2 | 20.00 | 14.99 |
3 | 20.00 | 15.01 |
(1)����Һ��Ũ��Ϊ____________mol/L��
(2)������ͭ��Ӧ��Ҫ����NOʱ�����Ũ�Ȳ�����____________mol/L��
��.(1)��3Cu+8H++2![]()
![]() 3Cu2++2NO��+4H2O
3Cu2++2NO��+4H2O
�ڽ�ͭ˿�������뿪Һ�棬��K��ͨ��N2һ��ʱ��
(2)����ʹͭ������ķ�Ӧ��ʱ��ʼ����ʱֹͣ
��.(1)0.15 (2)8
��������.��A�г�����ɫ����ʱ�������ѱ��ϡHNO3����Ӧ�����ӷ���ʽΪ3Cu+8H++2![]()
![]() 2Cu2++2NO��+4H2O���������IJ���Ӧ��ֹͣ��
2Cu2++2NO��+4H2O���������IJ���Ӧ��ֹͣ��
Ӧ���ɽ�ͭ˿�������뿪Һ�棬��K��ͨ��N2һ��ʱ�����ų�װ���е�NO���塣
��.(1)c(HNO3)=![]() =
=![]() =0.15 mol��L-1��
=0.15 mol��L-1��
(2)B������HNO3��0.15 mol��L-1��
3NO2+H2O![]() 2HNO3+NO
2HNO3+NO
0.045 mol 0.03 mol
Cu+4HNO3![]() Cu(NO3)2+2NO2+2H2O
Cu(NO3)2+2NO2+2H2O
0.09 mol 0.045 mol
ʣ��HNO3Ũ��Ϊ![]() =8 mol��L-1������������ͭ��Ӧ��Ҫ����NOʱ�����Ũ�Ȳ�����8 mol��L-1��
=8 mol��L-1������������ͭ��Ӧ��Ҫ����NOʱ�����Ũ�Ȳ�����8 mol��L-1��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�