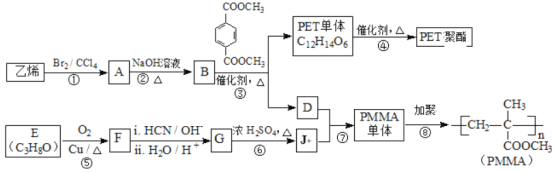
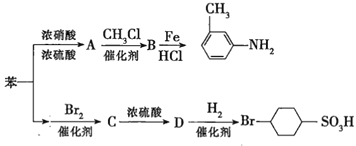
��Ŀ����
����Ŀ�������л��ϳɵ���Ҫ������
(1)��̬��ԭ�ӵļ۵����Ų�ʽ___________________________��
(2)���ͱ������Ṳ����ʵ���˱�ϩ��(CH2��CH��CH2OH)����ɫ��Ч�ϳɣ���ϩ����̼ԭ�ӵ��ӻ�������______________________����ȩ(CH3CH2CHO)���ϩ��(CH2��CH��CH2OH)��������ȣ�����ȩ�ȱ�ϩ���ķе�͵Ķ࣬����Ҫԭ����________________________��
(3)�ʻ���[Ni(CO)4]�����Ʊ��ߴ������ۡ��ʻ���[Ni(CO)4]��Ni��C��O �ĵ縺���ɴ�С��˳��Ϊ______��
(4)Ni2+ ���γɶ��������ӣ���[Ni(NH3)6]2+��[Ni(SCN)3]�� �ȡ�NH3�Ŀռ乹��Ϊ_______��
(5)��NiO��������ͼ��
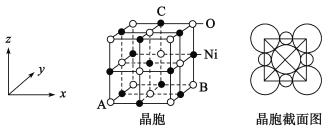
��������������ԭ���������AΪ(0,0,0)��BΪ(1,1,0)����Cԭ���������Ϊ_____________��
����֪�����������ܶ�dg/cm3��NA ���������ӵ�������ֵ����Ni2+ �뾶Ϊ________nm(�ô���ʽ��ʾ)����Ni�������ԭ��������59��
���𰸡�3d84s2 sp2��sp3 ��ϩ���з��Ӽ������� O>C>Ni ������ ![]()
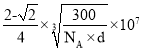
��������
(1)Ni��ԭ������Ϊ28�������Ų�ʽΪ1s22s22p63s23p63d84s2���۵����Ų�ʽ3d84s2��
(2)��ϩ��(CH2��CH��CH2OH)�е�����̼̼˫����̼ԭ�Ӽ۲���Ӷ���Ϊ3��Ϊsp2�ӻ�������̼ԭ�ӵļ۲���Ӷ���Ϊ4��Ϊsp3�ӻ�������̼ԭ�ӵ��ӻ�������sp2��sp3����ϩ��(CH2��CH��CH2OH)�������ǻ������ڷ��Ӽ���������Աȱ�ȩ(CH3CH2CHO)�ķе�ߣ�
(3)�縺����ԭ�ӶԼ��ϵ��ӵ������������ǽ�����Խǿ���縺��Խǿ������Ni��C��O �ĵ縺���ɴ�С��˳��ΪO��C��Ni��
(4)NH3��N���ӻ���ʽΪsp3��N��һ���µ��Ӷԣ�����NH3�Ŀռ乹��Ϊ�����Σ�
(5)��������������ԭ���������AΪ(0,0,0)��AΪԭ�㣬BΪ(1,1,0)�����߳�Ϊ1����Cԭ���������Ϊ![]() ��
��
�ڸ�����������������ͼ����֪������Խ��߳�ΪO2- �뾶��4������O2- �뾶Ϊr nm��������Ϊ2![]() r nm=2
r nm=2![]() r��10-7cm���������Ϊ
r��10-7cm���������Ϊ![]() cm3���������������У�O2-����Ϊ8��
cm3���������������У�O2-����Ϊ8��![]() +6
+6![]() =4����Ni2+����Ϊ12
=4����Ni2+����Ϊ12![]() +1=4��������һ������������Ϊ
+1=4��������һ������������Ϊ![]() g���������������ܶ�d =
g���������������ܶ�d = g/cm3 ����O2- �뾶r��
g/cm3 ����O2- �뾶r��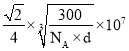 nm��������������������ͼ��֪��Ni2+��O2- �뾶֮��Ϊ����������һ�룬��
nm��������������������ͼ��֪��Ni2+��O2- �뾶֮��Ϊ����������һ�룬��![]() r nm����������Ϊ2
r nm����������Ϊ2![]() r nm����Ni2+�뾶Ϊ
r nm����Ni2+�뾶Ϊ![]() r��
r��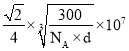 ��
��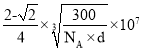 nm��
nm��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�