��Ŀ����
�״�����Ҫ����ɫ��Դ֮һ��Ŀǰ��ѧ����ˮú��(CO+H2)�ϳɼ״����䷴ӦΪ��
 ��H=-128.1kJ��mol-1���ش��������⣺
��H=-128.1kJ��mol-1���ش��������⣺
��1���÷�Ӧ�ǿ��淴Ӧ��Ϊʹ��ѧ��Ӧ���ʺ�CO��ת���ʶ�ͬʱ��ߵĴ�ʩ��_____________��д��������
��2�����º���������˵���ÿ��淴Ӧ��ƽ�����______________��
A��2v����H2����v�棨CH3OH��
B��n��CO����n��H2����n��CH3OH����1:2:1
C�����������ܶȲ���
D����������ƽ����Է�����������
��3�����������淴Ӧ�ں��º��ݵ��ܱ��������У���ʼʱ����������г���1molCO(g)��2molH2(g)��ʵ����H2��ƽ��ת�������¶�(T)��ѹǿ(P)�ı仯��ͼ��ʾ��

�÷�Ӧ�ġ�S____________0��ͼ�е�T1___________T2�������������������
��T1�µ���ƽ��״̬Aʱ�����������Ϊ2L����ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ_____________�����ﵽƽ��״̬Bʱ�������������V(B)��________L��
��4����֪��H2(g)ȼ���ȡ�H=-285.8kJ��mol-1����CO(g)ȼ���ȡ�H=-283.0kJ��mol-1����CH3OH(g)ȼ���ȵ��Ȼ�ѧ����ʽ��Ϊ____________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ͼװ����ȡ���ᴿ���ռ��±��е��������壨a��b��c��d��ʾ��Ӧ�����м�����Լ����ռ�װ����ȥ�������п��е���

���� | a | b | c | d | |
A |
| ϡ���� | ʯ��ʯ | ���� | Ũ |
B |
| Ũ���� |
| ���� | Ũ |
C |
| ���� | ��ʯ�� |
| ��ʯ�� |
D |
| ���� | � |
|
|


 Pb(Ac)2+(NH4)2SO4����Pb(Ac)2(����Ǧ)��Һ��ͨ��H2Sʱ���к�ɫ����PbS���������HAc���ɡ���ʾ�����Ӧ���й����ӷ���ʽ��ȷ����( )
Pb(Ac)2+(NH4)2SO4����Pb(Ac)2(����Ǧ)��Һ��ͨ��H2Sʱ���к�ɫ����PbS���������HAc���ɡ���ʾ�����Ӧ���й����ӷ���ʽ��ȷ����( )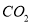
 ��Һ
��Һ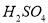
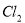
 ����
����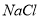 ��Һ
��Һ
 ��Һ
��Һ



 ɫ�������ɣ�Pb2++H2S=PbS��+2H+
ɫ�������ɣ�Pb2++H2S=PbS��+2H+