��Ŀ����
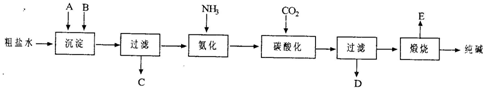
��ҵ��������Ĺ�������ʾ��ͼ���£�
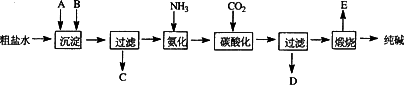
���������գ�
(1)����ˮ���������A��B������(������A��Դ��ʯ��Ҥ��)��д��A��B�Ļ�ѧʽ��
A__________________
B__________________
(2)ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����________��������________��________����ȴ�ᾧ��________����ɣ�
(3)��ҵ��������������У�̼�ữʱ������������___________________��
̼�ữʱû������̼���ƾ��壬��ԭ����___________________��
(4)̼�ữ����ˣ���ҺD����Ҫ�ijɷ���________(��д��ѧʽ)��������һ�ɷֵ������ӵľ��巽���ǣ�___________________��
(5)��������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��________��ҺD��ʯ��ˮǰ��Ҫ���ȣ�ԭ����__________________��
(6)��Ʒ�����к���̼�����ƣ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��__________________
(ע����ı���ʽ�����õ��йط��ŵĺ���)
�𰸣�

��ϰ��ϵ�д�
�����Ŀ