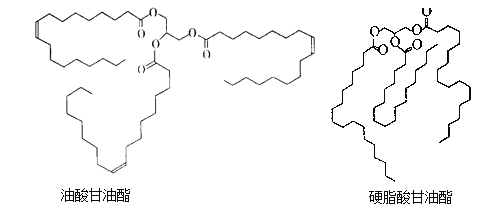
��Ŀ����
����Ŀ������ʽ̽������Ȫʵ������ѧ��ѧ��һ����Ҫ����ʵ�飬Ҳ��һ����Ȼ���������ԭ���Ǵ���ѹǿ��Ը�����ͼ���ش��������⣺

(1)ͼ��Ϊ��ѧ��ѧ�����õ���Ȫʵ��װ�á�����ƿ�г����������壬��ͷ�ιܼ��ձ���ʢ��Һ�塣��������в������γ���Ȫ����________(����ĸ����ͬ��)
A��HCl��H2O B��O2��H2O
C��NH3��H2O D��CO2��NaOH��Һ
(2)��ͼ�ҵ���ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����________��
A��Cu��ϡ����
B��NaHCO3��NaOH��Һ
C��CaCO3��ϡ����
D��NH4HCO3��ϡ����
(3)��ͼ�ҵ���ƿ���һˮ�ۣ���ƿ�м���ƾ���ˮ���м�����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʲ�������________��
A��Ũ���� B����ʯ��
C������� D���ռ�
(4)�����г�����������Ȫ����ɽ����ԭ��������________װ�õ�ԭ�����ơ�
(5)���ֻ�ṩ��ͼ��(��ƿ�ڳ���NH3)��װ�ã�������Ȫ�ķ�����__________________��
���𰸡�B D C �� ��ֹˮ�У�����(����ë����)����ƿ���ȣ������������ͣ��ϳ����������ڵĿ�����������ˮ�Ӵ�����������Ȫ����ƿ�ϸ�����ë����������ˮ��ʹ��ƿ���¶Ƚ��ͣ�ѹǿ��С���Ӷ�������Ȫ������ƿ��ͿĨ��ˮ�Ҵ��������Ҵ��ӷ���������ʹ��ƿ���¶Ƚ��ͣ�ѹǿ��С��������Ȫ
��������
��1�������γ���Ȫ����������ƿ�е���ѹ��С��Ҫ�������ɣ�
��2�����ݼ���������ܵ�����ƿ����ѹ����ԭ�����ܲ�����Ȫ��֪ʶ�������
��3����ͼ����ƿ���һˮ�ۣ�ƿ�м���ƾ���ˮ���м�����ˮ���ټ������������ʣ����Ҳ��������Ȫ���ͱ���Ҫ��ˮ���м�������ʺ���ʹ��ƿ�е��¶��������ߣ�
��4��ͼ��ԭ����ʹ��ƿ������ѹǿ��С�γ���Ȫ�������г�����������Ȫ����ɽ������ԭ����ͼ��ԭ�����ƣ�
��5������Ϣ��֪����ѹǿƽ�⣬����ѹǿ���γ���Ȫ��ʹ������ˮ��Ӧ������ƿ��ѹǿ��С����ѹ������ѹ���Ӷ��γ���Ȫ���Դ������
��1����װ�����γ���Ȫ����������ƿ�е���ѹ��С������ʹ��ƿ�е�������ٻ�������ƿ�е��¶ȼ�С��ԭ����ɣ�
A��HCl��H2O���Ȼ��⼫������ˮ�У��ܹ�ʹ��ƿ�е����������С�������ܹ��γ���Ȫ����A����
B��O2��H2O������������ˮ�����Բ���ʹ��ƿ�е������С�����γ���Ȫ����B��ȷ��
C��NH3��H2O��NH3��������ˮ�У��ܹ�ʹ��ƿ�е����������С�������ܹ��γ���Ȫ����C����
D��CO2��NaOH��Һ��Ѹ�ٷ�Ӧ���ܵ�����ƿ�е������С���������γ���Ȫ����D����
�ʴ�Ϊ��B��
��2��ͼ��������ƿ�м���������ܵ�����ƿ����ѹ�������γ���Ȫ��
A��Cu��ϡ�����Ӧ�����Բ��ܵ�����ƿ�е���ѹ������γ���Ȫ����A����
B��̼�����ƺ�NaOH��Һ�ܷ�Ӧ�������ܵ�����ƿ�е���ѹ���B����
C��CaCO3��ϡ���ᷴӦ��������ƣ������Ϊ�����ֹ�˷�Ӧ�������У��������γ���Ȫ����C����
D��̼�������ϡ���ᷴӦ���ɶ�����̼���壬�ܵ�����ƿ�е���ѹ��������ܹ��γ���Ȫ����D��ȷ��
�ʴ�Ϊ��D��
��3��A��Ũ��������ˮ�ų���������ʹ�ƾ��������������ƾ�������ʹ��ƿ��ѹǿ������ӣ������γ���Ȫ����A����
B����ʯ����ˮ��Ӧ�����������ƣ���Ӧ�ų��������ȣ�����ʹ��ƿ��ѹǿ���������ܹ��γ���Ȫ����B����
C����������Ҵ�����Ӧ�����ܵ�����ƿ��ѹǿ�������γ���Ȫ����C��ȷ��
D���ռ�����ˮ�зų�������������������ƿ���¶����ߣ�ѹǿ���������ܹ��γ���Ȫ����D����
�ʴ�Ϊ��C��
(4���Ƚ�ͼ��ͼ������װ�ã��Բ�����Ȫ��ԭ����������ͼA�Ǽ�С����ƿ�е�ѹǿ�γ���Ȫ�������г�����������Ȫ����ɽ������ԭ������ͨ�����������е�ѹǿ��ɵģ�������ͼ��ԭ�����ƣ�
�ʴ�Ϊ���ң�
(5)���ֻ�ṩ��ͼ��(��ƿ�ڳ���NH3)��װ�ã�������Ȫ�ķ����Ǵ�ֹˮ�У�����(����ë����)����ƿ���ȣ������������ͣ��ϳ����������ڵĿ�����������ˮ�Ӵ�����������Ȫ����ƿ�ϸ�����ë����������ˮ��ʹ��ƿ���¶Ƚ��ͣ�ѹǿ��С���Ӷ�������Ȫ������ƿ��ͿĨ��ˮ�Ҵ��������Ҵ��ӷ���������ʹ��ƿ���¶Ƚ��ͣ�ѹǿ��С��������Ȫ��

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�