��Ŀ����
����Ŀ����ʽ̼��ͭ��ʯ�ֽп�ȸʯ����֪��ʽ̼��ͭ��ĩ������ˮ�ʹ�����CuSO4��Һ��Na2CO3��Һ��Ӧ���Եõ���ʽ̼��ͭ��Ϊ��̽���÷�Ӧ�õ��ļ�ʽ̼��ͭ�Ļ�ѧʽ��ij��ͬѧ���������ʵ�飺
����ʽ̼��ͭ���Ʊ���
��ȡ12.5g��������ϸ���μ�4��ϡ���ᣬ������������ˮ�У���ֽ����õ�CuSO4��Һ�������м�������Na2CO3��Һ�����������������ɫ����Һ���ú���ˣ�����������ˮ����ˮ�Ҵ�ϴ����������ɫ���壬�����º�ɱ��á�
(1)����CuSO4��Һʱ���μ�ϡ�����������__________________________________��
(2)����ˮ�Ҵ�ϴ������ɫ�����Ŀ����_____________________________________��
��ʵ��̽����
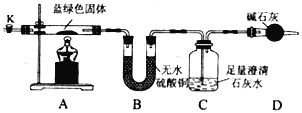
ͬѧ�����������װ�ã����Ƶõ�����ɫ�������ʵ�顣
�밴Ҫ����������⣺
(3)������װ�õ������ԣ�װ��ҩƷ��ʵ�鿪ʼǰ��ͨ��һ��ʱ������N2,Ȼ��رյ��ɼ�K,�ٵ�ȼA���ƾ��Ƽ��ȣ�����C�е��ܾ��ȵز������ݡ�ͨ��N2��������____________��N2�ĵ���ʽΪ____________��
(4)��ȼA���ƾ��ƺ��ܹ۲쵽��������_________________________________��
(5)װ��C����������Ӧ�����ӷ���ʽΪ_____________________________________��
(6)ͬѧ�Dz�������֪��Ksp[CaCO3]=2.8��10-9,Ksp[BaCO3]=5.1��10-9����������Ϊ��Ba(OH)2����Ca(OH)2�������ⶨ����ɫ����Ļ�ѧʽ����ã���ԭ����_________________(ѡ��������ĸ����)��
a��Ba(OH)2�ļ��Ա�Ca(OH)2ǿ
b��Ba(OH)2�ܽ�ȴ���Ca(OH)2,�ܳ������ CO2
c����ͬ������,CaCO3���ܽ�����Դ���BaCO3
d�����յ���CO2���ɵ�BaCO3����������CaCO3,�������С
(7)������ɫ��������ΪxCuCO3��yCu(OH)2��ȡ����������ɫ����10.84g,��������ȫ�ֽ��õ�8.00g���弰1.08gH2O���������ɫ����Ļ�ѧʽΪ___________��
���𰸡� ����Cu2+ˮ�⣬��ֹ�����Ƶ�CuSO4��Һ����� ��ȥ���������ˮ���������ھ���ĸ��� �ž�װ���еĿ����������ʵ����ɸ��� ![]() Ӳ�ʲ�����������ɫ������ɺ�ɫ��B�а�ɫ�������,C����Һ����� Ca2++2OH-+CO2=H2O+CaCO3�� bd 2CuCO3��3Cu(OH)2��3Cu(OH)2��2CuCO3��Cu5(OH)6(CO3)2
Ӳ�ʲ�����������ɫ������ɺ�ɫ��B�а�ɫ�������,C����Һ����� Ca2++2OH-+CO2=H2O+CaCO3�� bd 2CuCO3��3Cu(OH)2��3Cu(OH)2��2CuCO3��Cu5(OH)6(CO3)2
����������1��������ͭ�����������Cu2+�ᷢ��ˮ������������ͭ��Һ��ʱ��������������Ϊ������Cu2+��ˮ����
��2������ˮ�Ҵ�ϴ������ɫ�����Ŀ���ǽ���������ˮ��Ϊ�Ҵ����Ҵ�Զ��ˮ�ӷ������ҷе�������ȶ�Ҫ�ͣ������ں����ĸ������������Ƚ������ڵ����µõ�����ľ�����
��3��ͨ�뵪�����������ų�װ���ڵĿ�������Ϊ��ʵ����Ҫ�����ⶨ���ɵĶ�����̼��ˮ������������ԭװ���ڿ����к��еĶ�����̼��ˮ�������ʵ�����������š������ĵ���ʽΪ![]() ��
��
��4����ȼ�ƾ��ƺ��ʽ̼��ͭ���ȷֽ�Ϊ����ͭ��������̼��ˮ���������Թ۲쵽�������ǣ�A�й����Ϊ��ɫ��B�а�ɫ���������C����Һ�������
��5��װ��C���Ƕ�����̼����������������Һ��Ӧ�����ӷ���ʽΪ��Ca2++2OH-+CO2=H2O+CaCO3����
��6���������ƺ�������������ǿ�����˵�ĸ��ļ��Ը�ǿ�����Ҹ���Һ����Ҫ���������ն�����̼��ֻҪ�����㹻�Ϳ��ԣ�����Ҫ̫ǿ��ѡ��a������������������ˮ��������������ˮ����������������Һ��Ũ��Զ�����������ƣ������ͬ��ǰ���£��������������յĶ�����̼����һ��Զ�����������ƣ�ѡ��b��ȷ������Ksp��ֵ�����ߵ��ܽ�Ȳ��첻����ѡ��c���������յ����Ķ�����̼������Ӧ�õõ������ʵ�����̼�ᱵ��̼��Ƴ�������Ϊ̼�ᱵ����������һ������̼����������ⶨ������С��ѡ��d��ȷ�����Դ�Ϊbd��
��7��8.00g����ΪCuO�������ʵ���Ϊ0.1mol��1.08gˮ�����ʵ���Ϊ0.06mol���õ����������ӵ����ʵ���Ϊ0.12mol�����Եõ���ʽ̼��ͭ��Cu2+��OH- = 5:6�����õ����½����Cu5(OH)6(CO3)a�������ݻ��ϼ۴���֮��Ϊ0�������a=2�����Ըû�����ΪCu5(OH)6(CO3)2�����߸�дΪ2CuCO3��3Cu(OH)2��3Cu(OH)2��2CuCO3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�