��Ŀ����
Ŀǰ���������о�����һ�ָ��ܵ�ء�����-���أ����������ڵ��ơ���Ϊ��������Na+����Ħ�-Al2O3�մ����������ʣ���ӦʽΪ��2Na+xS��1��д���ŵ�ʱ�������ĵ缫��Ӧʽ��
������Ӧʽ��_____________________��������Ӧʽ��__________________________��
��2���øõ������Դ���е��M��NO3��m��Һʱ�����˵�ع���һ��ʱ�������46 g Na�����ص�ijһ������m g�������M�����ԭ������Ϊ��_______________����m��x��ʾ����
��3���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g Na��������������������Ϊ��___________L����״���£���������Һ��ˮϡ����2 L����Һ��pHΪ��_____________��
��1��xS+2e-=====Sx2- 2Na-2e-====2Na+
��2��![]()
��3��6.72 1
��������1������ܷ�ӦʽΪ2Na+xS![]() Na2Sx
Na2Sx
������Ӧʽ��xS+2e-====![]()
������Ӧʽ��2Na-2e-====2Na+
��2�������M�����ԭ������Ϊy,
�ڵ������У�����46 g Na������ʱ����·��ת�Ƶ��ӵ����ʵ���Ϊ2 mol��
���ʱ��������ӦʽΪ��
Mx++xe-====M
x mol y g
2 mol m g
��ã�y=![]()
��M�����ԭ������Ϊ![]() ��
��
��3���������Һʱ�������Ⱥ������з�Ӧ��
2Cl--2e-====Cl2��;4OH--4e-====2H2O+O2��
�����Ⱥ������з�Ӧ��
Cu2++2e-====Cu;2H++2e-====H2��
��������23 g Na����·����1 mol���ӷ���ת�ƣ���������������ת��1 mol���ӡ�
����������ʹCl-ȫ���ŵ����0.1 mol Cl2��0.2 mol e-������0.8 mol e-ʹOH-�ŵ����0.2 mol O2��
����������V��Cl2��+V��O2��=0.3 mol��22.4 L��mol-1=6.72 L
����������ʹCu2+ȫ���ŵ����0.4 mol e-������0.6 mol e-ʹ0.6 mol H+�ŵ硣
�Ƚ���������������0.6 mol H+��0.8 mol OH-�ֱ�ŵ磬����Һ�в���0.2 mol H+
![]()
pH=-1g c(H+)=1

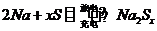
 C2Ex����д����طŵ�ʱ�������ĵ缫��Ӧʽ��������Ӧʽ��_______________________���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g C���ʡ������������������Ϊ��________L����״���£���������Һ��ˮϡ����2 L ����Һ��pHΪ��________��
C2Ex����д����طŵ�ʱ�������ĵ缫��Ӧʽ��������Ӧʽ��_______________________���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g C���ʡ������������������Ϊ��________L����״���£���������Һ��ˮϡ����2 L ����Һ��pHΪ��________��