جâؤ؟ؤعبف
»¯ر§ضذµؤؤ³ذ©شھثطتاسëةْأü»î¶¯أـ²»؟ة·ضµؤشھثط،£اë»ط´ًدآءذختجâ£؛
(1)NH4NO3تاز»ضضضطزھµؤ»¯ر§·تءد£¬ئنضذNش×سµؤشس»¯·½ت½·ض±ًتا________£¬NO3،ھµؤ؟ص¼ن¹¹ذحخھ____________،£
(2)A،¢B،¢Cبشھثطµؤش×سذٍتزہ´خشِ´َ£¬ثüأاش×سµؤ×îحâ²مµç×سإإ²¼¾ùخھ4s1،£
¢ظBشھثط»ùج¬ش×سµç×سإإ²¼ت½خھ___________________________________________،£
¢عAشھثطµ¥ضتµؤ¾§جه¶ر»ؤ£ذحخھ________(جî×ضؤ¸)£¬ئن؟ص¼نہûسأآتخھ__________،£
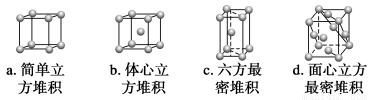
¢غاâشھثطسëCشھثط؟ةذخ³ةز»ضض؛ىة«»¯؛دخئن¾§جه½ل¹¹µ¥شھبçدآح¼،£شٍ¸أ»¯؛دخïµؤ»¯ر§ت½خھ__________(ذ،°×اٍ±يت¾H£¬ذ،؛عاٍ±يت¾C)،£
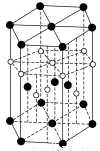
(3)زرضھµھ»¯إً(BN)µؤز»ضض¾§جه½ل¹¹سë½ً¸صت¯دàثئ£¬شٍB،ھN،ھBض®¼نµؤ¼ذ½اتا________£¬µھ»¯إًµؤأـ¶بخھ3.52 g،¤cm£3£¬شٍB،ھN¼üµؤ¼ü³¤تا________pm(ض»زھاَءذثمت½£¬²»±ط¼ئثم³ِتضµ£¬°¢·ü¼سµآآق³£تخھNA)،£
(1)sp3،¢sp2شس»¯،،ئ½أوب½اذخ
(2)¢ظ1s22s22p63s23p63d54s1»ٍ[Ar]3d54s1
¢عb،،68%
¢غCuH
(3)109،م28،ن،،
،¾½âخِ،؟(1)NH4+خھصثؤأوجه½ل¹¹£¬ثùزشNش×سµؤشس»¯·½ت½خھsp3£¬NO3،ھضذ؛¬سذ3¶ش¼غ²مµç×س¶ش£¬ئنہë×س¹¹ذحخھئ½أوب½اذخ£¬Nش×س²ةب،sp2شس»¯،£
(2)A،¢B،¢Cµؤ¼غµç×س¹¹ذح·ض±ًخھ4s1،¢3d54s1،¢3d104s1£¬ثùزشAخھK£¬BخھCr£¬CخھCu،£Kخھجهذؤء¢·½¶ر»£¬Cuخھأوذؤء¢·½×îأـ¶ر»£¬جهذؤء¢·½¶ر»µؤ؟ص¼نہûسأآتخھ68%£¬¶ّأوذؤء¢·½×îأـ¶ر»µؤ؟ص¼نہûسأآتخھ74%،£
¢غH( )،أ6،ء
)،أ6،ء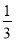 £«1£«3£½6
£«1£«3£½6
Cu( )،أ12،ء
)،أ12،ء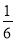 £«2،ء
£«2،ء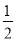 £«3£½6
£«3£½6
ئن»¯ر§ت½خھCuH،£
(3)BNµؤ»ù±¾½ل¹¹µ¥شھخھصثؤأوجه£¬ثùزشB،ھN،ھBض®¼نµؤ¼ذ½اخھ109،م28،ن،£ز»¸ِ½ً¸صت¯¾§°ûضذ؛¬سذCش×ستخھ8،ء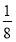 £«6،ء
£«6،ء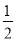 £«4£½8¸ِ£¬ثùزشز»¸ِBN¾§°ûضذ؛¬سذ4¸ِBش×س£¬4¸ِNش×س£¬ة辧°ûµؤہⳤخھa cm£¬شٍB،ھNµؤ¼ü³¤خھ
£«4£½8¸ِ£¬ثùزشز»¸ِBN¾§°ûضذ؛¬سذ4¸ِBش×س£¬4¸ِNش×س£¬ة辧°ûµؤہⳤخھa cm£¬شٍB،ھNµؤ¼ü³¤خھ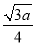 (خھجه¶ش½ادكµؤ
(خھجه¶ش½ادكµؤ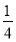 )،£
)،£
a3،¤3.52،ءNA£½4،ء25
a£½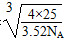
ثùزشئن¼ü³¤خھ pm،£
pm،£

 ر§ئع¸´د°ز»±¾ح¨ر§د°×ـ¶¯ش±ئعؤ©¼ستî¼ظرس±كبثأٌ³ِ°وةçدµءذ´ً°¸
ر§ئع¸´د°ز»±¾ح¨ر§د°×ـ¶¯ش±ئعؤ©¼ستî¼ظرس±كبثأٌ³ِ°وةçدµءذ´ً°¸ أ¢¹û½ج¸¨تî¼ظجىµطضطاى³ِ°وةçدµءذ´ً°¸
أ¢¹û½ج¸¨تî¼ظجىµطضطاى³ِ°وةçدµءذ´ً°¸