��Ŀ����
���������������������γ����������꣬�������γɹ⻯ѧ��������˱���Ժ��е�������ķ������д�����
(1)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�NO2+NO+2NaOH = 2NaNO2+H2O �ڸ÷�Ӧ�У���������_____________����ԭ����____________��
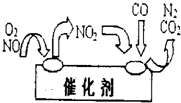
(2)����β���к���һ��������һ����̼���������������ʶԴ�����Ⱦ�ķ����ǰ�װ��ת������ʹ���Ƿ�Ӧ���ɵ����Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________��
(3)����Ҳ��������������������磬��������������������·�Ӧ��6NO2+8NH3 = 7N2+12H2O ��ij�����ŷŵ�β���ж�����������Ϊ0.5%�����������������1��103m3����״��������β������Ҫ����ǧ�˰�����
(1)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�NO2+NO+2NaOH = 2NaNO2+H2O �ڸ÷�Ӧ�У���������_____________����ԭ����____________��
(2)����β���к���һ��������һ����̼���������������ʶԴ�����Ⱦ�ķ����ǰ�װ��ת������ʹ���Ƿ�Ӧ���ɵ����Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________��
(3)����Ҳ��������������������磬��������������������·�Ӧ��6NO2+8NH3 = 7N2+12H2O ��ij�����ŷŵ�β���ж�����������Ϊ0.5%�����������������1��103m3����״��������β������Ҫ����ǧ�˰�����
(1) NO2��NO
(2)
(3)5.06 kg
(2)

(3)5.06 kg

��ϰ��ϵ�д�
�����Ŀ

 �������糧ȼú���̣��ͷų�������NOx��SO2���壮���������ȵ糧ͬʱ���������ѵ���������ʩ����Ϊ��Ч����ɫ����ȼú�糧���Իش��������⣺
�������糧ȼú���̣��ͷų�������NOx��SO2���壮���������ȵ糧ͬʱ���������ѵ���������ʩ����Ϊ��Ч����ɫ����ȼú�糧���Իش��������⣺