��Ŀ����
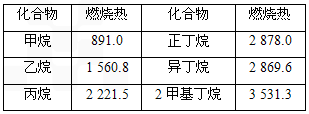
������һЩ������ȼ����(kJ/mol)���ݣ��ش��������⣺
|
������ |
ȼ���� |
������ |
ȼ���� |
|
���� |
891.0 |
������ |
2 878.0 |
|
���� |
1 560.8 |
�춡�� |
2 869.6 |
|
���� |
2 221.5 |
2������[��Դ:ѧ+��+��Z+X+X+K] |
3 531.3 |
��1�����ʵ�����Խ��Խ���ȶ����Ƚ������顢�춡������ȶ��ԣ�������________�춡��(�>����<��)��
��2��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��_________________________________
��3����ͬ������������̼����������Խ��ȼ�շų�������________(�Խ�ࡱ��Խ�١�����ͬ��)��
��1����
��2��C2H6��g��+7/2O2��g����2CO2��g��+3H2O��l�� �SH����1560.8 KJ/mol
��3��Խ��
����������1���������ȼ���ȴ�˵��������������ߣ�����ȶ���С���춡��ġ�
��2��ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�����������������ȼ���ȵ��Ȼ�ѧ����ʽΪC2H6��g��+7/2O2��g����2CO2��g��+3H2O��l�� �SH����1560.8 KJ/mol��
��3������ȼ���ȿ�֪����������ͬ�Ǽ���ų���������࣬�������к���������ߵģ���˵��̼����������Խ��ȼ�շų�������Խ�١�

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�| ������ | ȼ���� | ������ | ȼ���� |
| ���� | 891.0 | ������ | 2 878.0 |
| ���� | 1 560.8 | �춡�� | 2 869.6 |
| ���� | 2 221.5 | 2-������ | 3 531.3 |
��2��д������ȼ�յ��Ȼ�ѧ����ʽ��
��3����ͬ������������̼����������Խ��ȼ�շų�������
������һЩ������ȼ����(kJ/mol)���ݣ��ش��������⣺
| ������ | ȼ���� | ������ | ȼ���� |
| ���� | 891.0 | ������ | 2 878.0 |
| ���� | 1 560.8 | �춡�� | 2 869.6 |
| ���� | 2 221.5 | 2������ | 3 531.3 |
��1�����ʵ�����Խ��Խ���ȶ����Ƚ������顢�춡������ȶ��ԣ�������________�춡��(�>����<��)��
��2��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��_________________________________
��3����ͬ������������̼����������Խ��ȼ�շų�������________(�Խ�ࡱ��Խ�١�����ͬ��)��