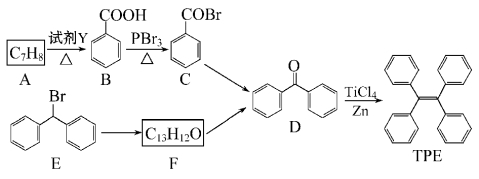
��Ŀ����
����Ŀ����ˮAlCl3���������������л��ϳɵĴ����ȡ���ҵ������������Al2O3��Fe2O3��Ϊԭ���Ʊ���ˮAlCl3�Ĺ����������¡�

��ʾ��2Fe(OH)3![]() Fe2O3 �� 3H2O
Fe2O3 �� 3H2O
��1�����ڿ�������ǿ�Ŀ���ʴ����ԭ���� ��
��2���Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽΪ ________________________��
��3����Na2SO3��Һ�ɳ�ȥ��ȴ���ų���β���е�Cl2����Ӧ�����Һ�ʼ��ԣ��˷�Ӧ�����ӷ���ʽΪ
_____________________��
��4��Ϊ�ⶨ�Ƶõ���ˮAlCl3��Ʒ��������FeCl3���Ĵ��ȣ���ȡ16.25 g��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�У����˳�����������ᆳϴ�ӡ����ա���ȴ�����أ���������Ϊ0.32 g��
��д���������ӹ������漰�����ӷ���ʽ�� �� ��
�ڲ�Ʒ��AlCl3����������Ϊ��ֻ�г�����ʽ������������ ��
���𰸡�����12����ÿ��2����
��1�������������ܵ�����Ĥ����ֹ�ڲ���������Ӵ�����ֹ��������
��2��Al2O3��3Cl2��3C![]() 2AlCl3��3CO
2AlCl3��3CO
��3��SO![]() ��2OH��+ Cl2 =SO
��2OH��+ Cl2 =SO![]() ��2Cl����H2O
��2Cl����H2O
��4����Fe3����3OH��=Fe(OH)3�� Al3����4OH��=AlO![]() ��2H2O
��2H2O
�� ��100%
��100%
��������
���������
��1�����ڿ�������ǿ�Ŀ���ʴ�ԣ�ԭ���������������ܵ�����Ĥ����ֹ�ڲ���������Ӵ�����ֹ����������
��2�����ݹ������̿�֪�Ȼ�¯�IJ������ȴ���������Ʊ���ˮAlCl3��˵���Ȼ�¯�IJ����к���A1C13��������β������CO������Al2O3��C12��C��Ӧ������A1C13��CO����Ӧ����ʽΪA12O3+3C12+3C![]() 2A1C13+3CO��
2A1C13+3CO��
��3��Cl2��ǿ�����ԣ���SO32-����ΪSO42-����������ԭΪ2C1-����Ӧ���ӷ���ʽΪSO32-+C12+H2O�TSO42-+2C1-+2H+��
��4�������ӹ����������������������ӽ�ϳ��������������������������������ӽ�ϳ�ƫ�������ˮ�����ӷ���ʽΪ��Fe3++3OH-=Fe(OH)3����Al3++4OH-=AlO2-+2H2O��
��������ԭ���غ㣬Fe2O3~2FeCl3�ɵ�FeCl3����Ϊ![]() ��2��162.5����AlCl3��Ʒ�Ĵ���Ϊ
��2��162.5����AlCl3��Ʒ�Ĵ���Ϊ ��100%��
��100%��

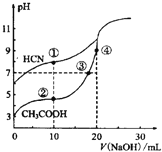
����Ŀ����50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
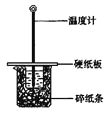
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ ������ƫ��ƫС����Ӱ������
��3�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� ��������ȡ�����������������к��� ��������ȡ��������������������
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� ������ƫ��������ƫС��������Ӱ��������ͬ�� ����KOH����NaOH���ⶨ����� __ __
��5�����Ǽ�¼��ʵ���������£�
ʵ �� �� Ʒ | �� Һ �� �� | �к�����H | |||
t1 | t2 | ||||
�� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� | |
�� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� | |
��֪��Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ�Ϊ1g��cm-3��
����������ϱ���
������ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ�� ��