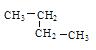��Ŀ����
����Ŀ����1��������з�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ������������ƽ����ʽ��
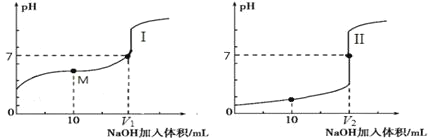
��ʵ��������Ȳ��_________
��������Һ��ͨ�������̼���壺________
�ۼױ��������ڹ��������·�Ӧ����һ��ȡ�����___
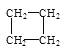
��2���л���A�Ľṹ��ʽΪ

����A�ǵ�ϩ���������ӳɺ�IJ����õ�ϩ��������____�ֽṹ�������������칹����
����A��Ȳ���������ӳɺ�IJ�����Ȳ��������____�ֽṹ��
����A��һ��ͬ���칹��ֻ����һ��ϩ������õ����Ҹ�ϩ����һ���dz��ԳƵķ��ӹ��ͣ���˳�������ֽṹ��
д��A�ĸ���ͬ���칹�壨����˳���칹���Ľṹ��ʽ______��_____��
��3��C5H12O��ͬ���칹����____�֣��������ڴ������ܱ�������ȩ����____�֣�����ȥH2O����ϩ�Ĵ���_____�֡�
���𰸡�CaC2+2H2O��Ca(OH)2+C2H2�� 
 5 1
5 1 ![]()
![]() 14 4 7
14 4 7
��������
��1����ʵ�������õ�ʯˮ���Ƶ���Ȳ����ѧ����ʽΪCaC2+2H2O��Ca(OH)2+C2H2����
��������Һ��ͨ�������̼����õ����Ӻ�NaHCO3�������ѧ����ʽΪ ��
��
�ۼױ��������ڹ��������·�Ӧ��ֻ�м��ϵ���ԭ�ӿɱ�ȡ���������ѧ����ʽΪ ��
��
��2����A�ǵ�ϩ���������ӳɺ�IJ��˫��ֻ�����������ڵ�̼ԭ��֮����������̼ԭ��������ԭ�ӣ���������̼���ṹΪ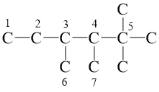 ��5��̼ԭ����û����ԭ�ӣ����̼ԭ��������̼ԭ��֮�䲻���γ�̼̼˫�������γ�˫��λ���У�1��2֮�䣬2��3֮�䣬3��4֮�䣬3��6֮�䣬4��7֮�䣬�ʸ�ϩ������5�ֽṹ��
��5��̼ԭ����û����ԭ�ӣ����̼ԭ��������̼ԭ��֮�䲻���γ�̼̼˫�������γ�˫��λ���У�1��2֮�䣬2��3֮�䣬3��4֮�䣬3��6֮�䣬4��7֮�䣬�ʸ�ϩ������5�ֽṹ��
������Ȳ���������ӳɷ�Ӧ��ԭ������֪����������������̼ԭ���Ͼ���2����ԭ�ӵ�̼ԭ�Ӽ��Ƕ�ӦȲ������̼̼������λ�ã���������̼���ṹΪ ���������γ�����λ��ֻ��1��2֮�䣬�ʸ�Ȳ����1�ֽṹ��
���������γ�����λ��ֻ��1��2֮�䣬�ʸ�Ȳ����1�ֽṹ��
����A��һ��ͬ���칹��ֻ����һ��ϩ������õ����Ҹ�ϩ����һ���dz��ԳƵķ��ӹ��ͣ�ϩ��Ϊ(CH3)3CCH=CHC(CH3)3����A��ͬ���칹��Ϊ(CH3)3CCH2CH2C(CH3)3����ϩ����˳���칹��Ľṹ��ʽ��![]() ��
��![]() ��
��
��3����Ϊ��������ʽ�ɸ�дΪC5H11-OH�����-C5H11��8�֣������Ҳ��8�ֽṹ���ֱ���CH3CH2CH2CH2CH2OH��CH3CH2CH2CHOHCH3��CH3CH2CHOHCH2CH3��HOCH2CH(CH3)CH2CH3��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3��(CH3)2CHCH2CH2OH��(CH3)3CCH2OH����Ϊ�ѣ��ܹ�6�֣��ֱ�ΪCH3OCH2CH2CH2CH3��CH3CH2OCH2CH2CH3��CH3OCH(CH3)CH2CH3��(CH3)2CHOCH2CH3��(CH3)2CHCH2OCH3��(CH3)3COCH3���������ʽΪC5H12O��ͬ���칹�干14�֡�������RCH2OH������ʽ�ӿɱ�������ȩ�������������������CH3CH2CH2CH2CH2OH��HOCH2CH(CH3)CH2CH3��(CH3)2CHCH2CH2OH��(CH3)3CCH2OH���ϼ�Ϊ4�֡������ǻ���λ̼������ԭ�ӿ���ȥ�����������������CH3CHspan>2CH2CH2CH2OH��CH3CH2CH2CHOHCH3��CH3CH2CHOHCH2CH3��HOCH2CH(CH3)CH2CH3��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3��(CH3)2CHCH2CH2OH���ܹ�7�֡�

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�����Ŀ���±��������Ԫ�����ڱ���һ����Ԫ�أ��ش��������⣺
1 | ||||||||||||||||||
2 | A | B | C | |||||||||||||||
3 | D | E | F | G | H | I | J | K | ||||||||||
4 | M | |||||||||||||||||
��1�����ϱ�����ĸ�����12��Ԫ���У���ѧ��������õ���____����Ԫ�ط��ű�ʾ����ͬ������������ǿ����___����ϡ�����������������ԭ�Ӱ뾶��С����____�����ڹ���Ԫ�ص���___���ÿո��ñ��е���ĸ��ʾ����
��2��J���⻯��ĵ���ʽΪ____������������Ӧ��ˮ����Ļ�ѧʽΪ___��
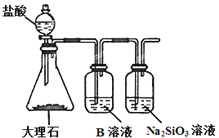
��3��Ϊ�Ƚ�Ԫ��A��G�ķǽ�����ǿ��������ͼ��ʾ��װ�ý���ʵ�飨�г���������ȥ��װ�����������ã�����ҺBӦ��ѡ��_____��Һ��������_____����˵��A��G�ǽ�����ǿ���Ļ�ѧ����ʽ��______��