��Ŀ����
��100��ʱ����0.100mol��N2O4�������1L��յ��ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ����±���
|
ʱ��/s
Ũ��mol��L-1 |
0 |
20 |
40 |
60 |
80 |
100 |
|
c��N2O4��mol��L-1 |
0.100 |
c1 |
0.050 |
c3 |
a |
b |
|
c��NO2��/mol��L-1 |
0.000 |
0.060 |
c2 |
0.120 |
0.120 |
0.120 |
����գ�
��1���ﵽƽ��ʱN2O4��ת����Ϊ %������c2 c3 ��a b ��ѡ���������������������
��2��20s��������������Ũ��c1= mol��L-1����0s~20s��������������ƽ����Ӧ����Ϊ mol����L��s��-1����
��3��������ͬ���������������������Ƕ�����������Ҫ�ﵽ����ͬ����ƽ��״̬��������������ʼŨ���� mol��L-1��
���𰸡�
(1)60��>��= (2)0.070��0.0015 (3)0.200
����������

��ϰ��ϵ�д�
�����Ŀ
��100��ʱ����0.40molNO2�������2L�ܱ������У�ÿ��һ��ʱ��Ը����������ʽ��в������õ����������±���
|
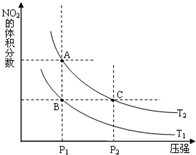 �����γɶ����������NO��NO2��N2O4�ȣ�NO2��N2O4�����ת����
�����γɶ����������NO��NO2��N2O4�ȣ�NO2��N2O4�����ת������1���Է�Ӧ2NO2��g��?N2O4��g����H=-57.2kJ?mol-1
�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��T1
��A��C���������ƽ����Է���������A
��2����100��ʱ����0.40mol��NO2�������2L���ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ�������ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n��NO2��/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n��N2O4��/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
�ڸ�������ƽ�ⳣ��K��ֵΪ
���������������䣬���м��ܼӿ�����Ӧ�����������NO2ת���ʵĴ�ʩ��
��������ͬ����������������������N2O4���壬Ҫ�ﵽ����ͬ����ƽ��״̬��N2O4����ʼ�����ʵ�����
���������������䣬ֻ��������Ϊ�������ĺ�ѹ��������ƽ��ʱN2O4����
 ��100��ʱ����0.40mol���������������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�
��100��ʱ����0.40mol���������������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�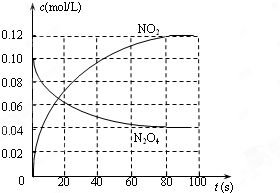
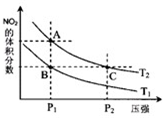 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�