��Ŀ����
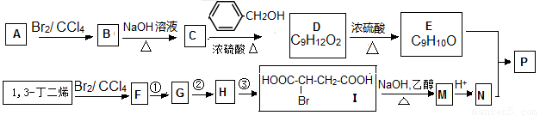
���۷��ɸĽ��л��߷��ӻ���������ʣ��߷��Ӿۺ���P�ĺϳ�·�����£�
��֪��
R-CH2O
R-COOH ��R��ʾ����������
R-OH+R��-OH
R-O-R��+H2O ��R��R���ʾ������
 nCH=CH+nCH=CH
nCH=CH+nCH=CH
R1��R4��ʾ���������
��1��A�Ľṹ��ʽΪ
��2��C������Ϊ
��3��I��F���١��ۺϳɣ�F��ʹ��ˮ��ɫ
a���ٵĻ�ѧ��Ӧ����ʽ��
 ��
��
b���ڵķ�Ӧ�Լ���
��4������˵����ȷ����
a��C����ˮ������������� b��A��1��3-����ϩ��Ϊͬϵ��
c��I����Mʱ��1mol I�������3mol NaOH d��N������˳���칹��
��5���߾���P����ˮ�ԣ�����E�γɵľۺ���
��6��E��N�������ʵ���֮��Ϊ1��1������������P��P�Ľṹ��ʽΪ
 ��
��
��6��E�ж���ͬ���칹�壬���������������칹����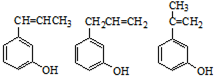
 ��
��
a��������ֻ��һ�ֻ�״�ṹ b��������������ȡ���� c.1mol���л�������ˮ��Ӧʱ������4mol Br2��

��֪��
R-CH2O
| KMnO3/H+ |
R-OH+R��-OH
| Ũ���� |
| �� |
 nCH=CH+nCH=CH
nCH=CH+nCH=CH| ���� |
��1��A�Ľṹ��ʽΪ
CH2=CH2
CH2=CH2
����2��C������Ϊ
�Ҷ���
�Ҷ���
����3��I��F���١��ۺϳɣ�F��ʹ��ˮ��ɫ
a���ٵĻ�ѧ��Ӧ����ʽ��


b���ڵķ�Ӧ�Լ���
HCl
HCl
c���۵ķ�Ӧ������������Ӧ
������Ӧ
����4������˵����ȷ����
ac
ac
��a��C����ˮ������������� b��A��1��3-����ϩ��Ϊͬϵ��
c��I����Mʱ��1mol I�������3mol NaOH d��N������˳���칹��
��5���߾���P����ˮ�ԣ�����E�γɵľۺ���
ǿ
ǿ
���ǿ������������6��E��N�������ʵ���֮��Ϊ1��1������������P��P�Ľṹ��ʽΪ


��6��E�ж���ͬ���칹�壬���������������칹����
3
3
�֣�������˳���칹����д������һ���칹��Ľṹ��ʽ

a��������ֻ��һ�ֻ�״�ṹ b��������������ȡ���� c.1mol���л�������ˮ��Ӧʱ������4mol Br2��
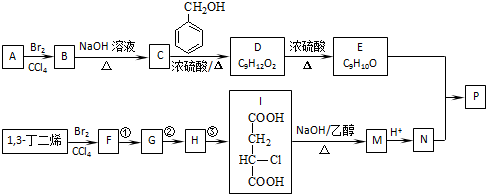
������A���巢���ӳɷ�Ӧ����B��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��C�ͱ��״���Ӧ����D����A��B��C��̼ԭ�Ӹ�����ͬ������D�ķ���ʽ֪��A�к�������̼ԭ�ӣ���A����ϩ��B��1��2-�����飬C���Ҷ��������������Ϣ֪��D�Ľṹ��ʽΪ��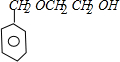 ��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ��
��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ��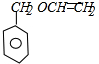 ������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ��
������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ�� ��
��
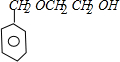 ��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ��
��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ�� ������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ��
������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ�� ��
������⣺A���巢���ӳɷ�Ӧ����B��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��C�ͱ��״���Ӧ����D����A��B��C��̼ԭ�Ӹ�����ͬ������D�ķ���ʽ֪��A�к�������̼ԭ�ӣ���A����ϩ��B��1��2-�����飬C���Ҷ��������������Ϣ֪��D�Ľṹ��ʽΪ��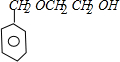 ��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ��
��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ�� ������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ��
������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ�� ��
��
��1��ͨ�����Ϸ���֪��A����ϩ����ṹ��ʽΪ��CH2=CH2���ʴ�Ϊ��CH2=CH2��
��2��C���Ҷ������ʴ�Ϊ���Ҷ�����
��3��a����1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����Ӧ����ʽΪ��
 ��
��
�ʴ�Ϊ�� ��
��
b��G���Ȼ��ⷢ���ӳɷ�Ӧ����H�����Ԣڵķ�Ӧ�Լ���HCl���ʴ�Ϊ��HCl��
c��H�����Ը��������������I�����Ԣ۵ķ�Ӧ������������Ӧ���ʴ�Ϊ��������Ӧ��
��4��a��C���Ҷ���������ˮ������������ܣ�����ȷ��
b��A����ϩ����ϩ��1��3-����ϩ�ṹ��ͬ�����Բ���Ϊͬϵ��ʴ���
c��I����Mʱ��1mol I�������3mol NaOH������ȷ��
d��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��N����˳���칹�壬�ʴ���
��ѡac��
��5��P�Ľṹ��ʽΪ ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ P�к�����ˮ����E������ˮ�������Ը߾���P����ˮ�Ա���E�γɵľۺ���ǿ���ʴ�Ϊ��ǿ��
P�к�����ˮ����E������ˮ�������Ը߾���P����ˮ�Ա���E�γɵľۺ���ǿ���ʴ�Ϊ��ǿ��
��6��ͨ�����Ϸ���֪��P�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6��E�Ľṹ��ʽΪ�� ��E�ж���ͬ���칹�壬E��ͬ���칹���������������a��������ֻ��һ�ֻ�״�ṹ˵��ֻ��������b��������������ȡ������c.1mol���л�������ˮ��Ӧʱ������4mol Br2��˵������һ��˫�����ұ����Ϻ��з��ǻ�����������3����ԭ�ӣ������������E��ͬ���칹��Ϊ��
��E�ж���ͬ���칹�壬E��ͬ���칹���������������a��������ֻ��һ�ֻ�״�ṹ˵��ֻ��������b��������������ȡ������c.1mol���л�������ˮ��Ӧʱ������4mol Br2��˵������һ��˫�����ұ����Ϻ��з��ǻ�����������3����ԭ�ӣ������������E��ͬ���칹��Ϊ�� �����������֣�
�����������֣�
�ʴ�Ϊ��3�� ��
��
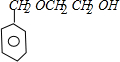 ��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ��
��D������ȥ��Ӧ����E����E�Ľṹ��ʽΪ�� ������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ��
������I�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����F 1��4-����-2-��ϩ��1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����G��G�Ľṹ��ʽΪ��HOCH2CH=CHCH2OH��G���Ȼ��ⷢ���ӳɷ�Ӧ����H��H�Ľṹ��ʽΪ��HOCH2CH2CHClCH2OH��H�����Ը��������������I��I���������ƵĴ���Һ������ȥ��Ӧ����M��M�Ľṹ��ʽΪ��NaOOCCH=CHCOONa��Ȼ���ữ�õ�N��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��E��N��Ӧ����P����P�Ľṹ��ʽΪ�� ��
����1��ͨ�����Ϸ���֪��A����ϩ����ṹ��ʽΪ��CH2=CH2���ʴ�Ϊ��CH2=CH2��
��2��C���Ҷ������ʴ�Ϊ���Ҷ�����
��3��a����1��4-����-2-��ϩ���������Ƶ�ˮ��Һ����ȡ����Ӧ����Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
��b��G���Ȼ��ⷢ���ӳɷ�Ӧ����H�����Ԣڵķ�Ӧ�Լ���HCl���ʴ�Ϊ��HCl��
c��H�����Ը��������������I�����Ԣ۵ķ�Ӧ������������Ӧ���ʴ�Ϊ��������Ӧ��
��4��a��C���Ҷ���������ˮ������������ܣ�����ȷ��
b��A����ϩ����ϩ��1��3-����ϩ�ṹ��ͬ�����Բ���Ϊͬϵ��ʴ���
c��I����Mʱ��1mol I�������3mol NaOH������ȷ��
d��N�Ľṹ��ʽΪ��HOOCCH=CHCOOH��N����˳���칹�壬�ʴ���
��ѡac��
��5��P�Ľṹ��ʽΪ
 ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ P�к�����ˮ����E������ˮ�������Ը߾���P����ˮ�Ա���E�γɵľۺ���ǿ���ʴ�Ϊ��ǿ��
P�к�����ˮ����E������ˮ�������Ը߾���P����ˮ�Ա���E�γɵľۺ���ǿ���ʴ�Ϊ��ǿ����6��ͨ�����Ϸ���֪��P�Ľṹ��ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����6��E�Ľṹ��ʽΪ��
 ��E�ж���ͬ���칹�壬E��ͬ���칹���������������a��������ֻ��һ�ֻ�״�ṹ˵��ֻ��������b��������������ȡ������c.1mol���л�������ˮ��Ӧʱ������4mol Br2��˵������һ��˫�����ұ����Ϻ��з��ǻ�����������3����ԭ�ӣ������������E��ͬ���칹��Ϊ��
��E�ж���ͬ���칹�壬E��ͬ���칹���������������a��������ֻ��һ�ֻ�״�ṹ˵��ֻ��������b��������������ȡ������c.1mol���л�������ˮ��Ӧʱ������4mol Br2��˵������һ��˫�����ұ����Ϻ��з��ǻ�����������3����ԭ�ӣ������������E��ͬ���칹��Ϊ�� �����������֣�
�����������֣��ʴ�Ϊ��3��
 ��
�����������⿼�����л�����ƶϼ��ϳɣ����������Ϣ�����������ϵķ������з������ע��̼̼˫���ױ����Ը������������������F����I�Ĺ����У�Ӧ�ȼӳɺ�������Ϊ�״��㣮

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ