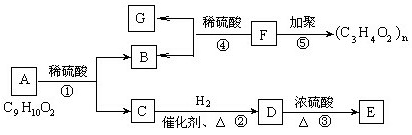
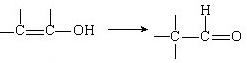��Ŀ����
��չ��̼���ã�������̼��ᣮ��ѧ������������Թ�ҵ�����е�CO2Ϊԭ�ϣ���CuO��ZnO�����Ϊ�������䷴ӦΪ��CO2+3H2?CH3OH+H2O��
��1��ij�¶��£������ΪlL���ܱ������г���lmolCO2��4molH2�����CO2��CH3OH��g����Ũ����ʱ��仯�磨��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬�״���ƽ����Ӧ����v��CH3OH��=______��������ת����Ϊ______��
��2�����³�ѹ����֪���з�Ӧ�������仯�磨��ͼ��ʾ��

д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��______���÷�Ӧ�ġ�S______0�������������=��������Ӧ�ﵽƽ���Ҫʹ��ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ��______������ţ���
A����С��Ӧ�����B�������¶�C������������ͨ��CO2D��ʹ�ú��ʵĴ���
��3����ʵ�������з��֣����ż״������ɣ�������������CO�ȸ�������֣���CO2��ת���ʡ��״���CO�ĺ����������������ڷ�Ӧ��¯�ڵ��������ʡ�����CuO����������Ӱ�죮ͨ��ʵ��ֱ�õ���ͼ����
����ͼ���ã������״������������������Ϊ______L?h-1��
����֪��������û��CuO��ֻ�е����ZnOʱ����Ӧ�������˵��Ϊʲô��ѡ�����ZnO��ԭ______������ͼ�������жϣ�����CuO�������������Ϊ______%��
��1��ij�¶��£������ΪlL���ܱ������г���lmolCO2��4molH2�����CO2��CH3OH��g����Ũ����ʱ��仯�磨��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬�״���ƽ����Ӧ����v��CH3OH��=______��������ת����Ϊ______��
��2�����³�ѹ����֪���з�Ӧ�������仯�磨��ͼ��ʾ��

д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��______���÷�Ӧ�ġ�S______0�������������=��������Ӧ�ﵽƽ���Ҫʹ��ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ��______������ţ���
A����С��Ӧ�����B�������¶�C������������ͨ��CO2D��ʹ�ú��ʵĴ���
��3����ʵ�������з��֣����ż״������ɣ�������������CO�ȸ�������֣���CO2��ת���ʡ��״���CO�ĺ����������������ڷ�Ӧ��¯�ڵ��������ʡ�����CuO����������Ӱ�죮ͨ��ʵ��ֱ�õ���ͼ����
����ͼ���ã������״������������������Ϊ______L?h-1��
����֪��������û��CuO��ֻ�е����ZnOʱ����Ӧ�������˵��Ϊʲô��ѡ�����ZnO��ԭ______������ͼ�������жϣ�����CuO�������������Ϊ______%��
��1��10min����ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.75mol/L���ӷ�Ӧ��ʼ��ƽ�⣬�״���ƽ����Ӧ����v��CH3OH��=
0.075mol/L?min�����ݻ�ѧ����ʽCO2+3H2?CH3OH+H2O������õ���Ӧ���������ʵ���=0.75mol/L��1L��3=2.25mol������ת����=
��100%=56.25%��
�ʴ�Ϊ��0.075mol/��L��min����56.25%��
��2��ͼ2���Ȼ�ѧ����ʽ��CO��g��+H2O��l��=CO2��g��+H2��g����H=-41KJ/mol��
��CO��g��+2H2��g��=CH3OH��l����H=-��510-419��KJ/mol=-91KJ/mol��
���ݸ�˹���ɢ�-�ٵõ�������̼�������Ʊ��״����Ȼ�ѧ����ʽ��CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-50KJ/mol����Ӧǰ�����������С����S��0��Ҫʹ��ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ���ݻ�ѧƽ���ƶ�ԭ��������
A����С��Ӧ�����������ѹǿ��ƽ��������У���A���ϣ�
B����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ��������У���B�����ϣ�
C������������ͨ��CO2������Ӧ��Ũ��ƽ��������У���C���ϣ�
D��ʹ�ú��ʵĴ����ı䷴Ӧ���ʲ��ı仯ѧƽ�⣬��D�����ϣ�
��AC��ȷ��
�ʴ�Ϊ��CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-50KJ/mol������AC��
��3��ͼ����������״�����������������ʣ�������̼ת������״����������ͼ�����ݿ�֪�������������3600���϶����ԣ�ʹ�õ����ZnOʱ��Ӧ������Ȼ��죬������ͼ��֪��CO2ת���ʡ�CH3OH���ʾ����ͣ�ͼ�������������̼ת������״��������ʱ����CuO���������������50%��
�ʴ𰸣�3600���϶����ԣ�ʹ�õ����ZnOʱ��Ӧ������Ȼ��죬������ͼ��֪��CO2ת���ʡ�CH3OH���ʾ����ͣ�ʵ��������û�����壬�ʲ����ã�50%��
| 0.75mol/L |
| 10min |
| 2.25mol |
| 4mol |
�ʴ�Ϊ��0.075mol/��L��min����56.25%��
��2��ͼ2���Ȼ�ѧ����ʽ��CO��g��+H2O��l��=CO2��g��+H2��g����H=-41KJ/mol��
��CO��g��+2H2��g��=CH3OH��l����H=-��510-419��KJ/mol=-91KJ/mol��
���ݸ�˹���ɢ�-�ٵõ�������̼�������Ʊ��״����Ȼ�ѧ����ʽ��CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-50KJ/mol����Ӧǰ�����������С����S��0��Ҫʹ��ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ���ݻ�ѧƽ���ƶ�ԭ��������
A����С��Ӧ�����������ѹǿ��ƽ��������У���A���ϣ�
B����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ��������У���B�����ϣ�
C������������ͨ��CO2������Ӧ��Ũ��ƽ��������У���C���ϣ�
D��ʹ�ú��ʵĴ����ı䷴Ӧ���ʲ��ı仯ѧƽ�⣬��D�����ϣ�
��AC��ȷ��
�ʴ�Ϊ��CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-50KJ/mol������AC��
��3��ͼ����������״�����������������ʣ�������̼ת������״����������ͼ�����ݿ�֪�������������3600���϶����ԣ�ʹ�õ����ZnOʱ��Ӧ������Ȼ��죬������ͼ��֪��CO2ת���ʡ�CH3OH���ʾ����ͣ�ͼ�������������̼ת������״��������ʱ����CuO���������������50%��
�ʴ𰸣�3600���϶����ԣ�ʹ�õ����ZnOʱ��Ӧ������Ȼ��죬������ͼ��֪��CO2ת���ʡ�CH3OH���ʾ����ͣ�ʵ��������û�����壬�ʲ����ã�50%��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 C(g)+ D(g)�Ѵﵽƽ����� ( )
C(g)+ D(g)�Ѵﵽƽ����� ( )