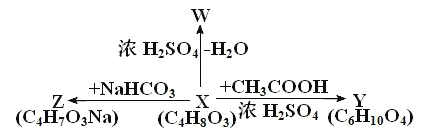
��Ŀ����
����Ŀ����CO2���ۺ����ÿ���ʵ�־�����ᷢչ����̬��������˫Ӯ���Դٽ���̼���Ĺ���������Ҫ���塣�ش��������⣺
��1����֪����C��ȼ����Ϊ393.5kJ��mol-1
��H2��ȼ����Ϊ286kJmol-1
��H2O(g)=H2O(l) ��H=��44.0kJmol-1
��CO2(g)+2H2(g)![]() C(s)+2H2O(g)��H=___kJmol��1��
C(s)+2H2O(g)��H=___kJmol��1��
��2��CO2�ϳɼ״���̼���ŵ�һ����Ҫ��������1L�����ܱ������м���1molCO2��amolH2�����ʵ��Ĵ��������£�������Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H=��48.7kJmol-1��ƽ��ʱ���������CH3OH��������������������ʵ�����ϵ��ͼ1��ʾ��
CH3OH(g)+H2O(g) ��H=��48.7kJmol-1��ƽ��ʱ���������CH3OH��������������������ʵ�����ϵ��ͼ1��ʾ��
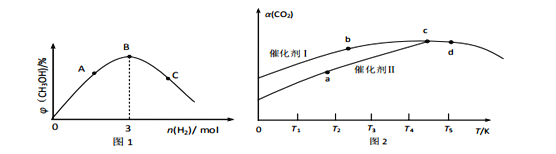
�������ڸ÷�Ӧ�Է����е�������___���������¸�ѹ�������µ�ѹ���������µ�ѹ������
��ͼ1�� A��B��C�����Ӧ����ϵ��CO2��ƽ��ת����������___������ĸ�����ж�������___��
��3������ CO2����������ϩ����ӦΪ 2CO2(g)+6H2(g)![]() C2H4(g)+4H2O(g) ��H��
C2H4(g)+4H2O(g) ��H��
��1L�����ܱ������У�������1molCO2��3molH2���ֱ�ѡ�����ִ�������Ӧ������ͬʱ����CO2ת����[��(CO2)]�淴Ӧ�¶ȵı仯��ͼ2��ʾ��
��ʹ�ô�����ʱ��Ӧ�Ļ��___������������������������ʹ�ô�����
��c��d�����ƽ�ⳣ����С��ϵΪ��Kc___Kd�����������������������������ж�������___��
��4�������£������ü�����Һ����CO2����100mL0.3mol��L-1Na2CO3��Һ��ȫ����0.88gCO2���壨������Һ����仯����������Һ�У�c(HCO3-)+c(CO32-)+c(H2CO3)=___molL-1��
���𰸡�-90.5 ���¸�ѹ C �ں����ܱ������У�CO2��Ũ�Ȳ��䣬����H2��Ũ�ȣ�ƽ�������ƶ���CO2��ת�������� ���� �� ƽ��������¶����ߣ�CO2��ת���ʽ��ͣ�����Ӧ���ȣ�K���¶����߶���С 0.5
��������
(1)C(s)��H2(g)��ȼ���ȷֱ���393.5kJmol-1 ��285.8kJmol-1 ����C(s)+O2(g)=CO2(g)��H=-393.5kJmol-1����H2(g)+![]() O2(g)=H2O(l)��H=-285.8kJmol-1����H2O(l)�TH2O(g)��H2=+44.0kJmol-1�����ݸ�˹���ɣ�����2-��+����2�ɵ�CO2(g)+2H2(g)
O2(g)=H2O(l)��H=-285.8kJmol-1����H2O(l)�TH2O(g)��H2=+44.0kJmol-1�����ݸ�˹���ɣ�����2-��+����2�ɵ�CO2(g)+2H2(g)![]() C(s)+2H2O(g)���ɴ˼�����H��
C(s)+2H2O(g)���ɴ˼�����H��
(2)���Է����е��ж���������H-T��S��0���÷�Ӧ��H��0����S��0��
�����������ʵ���Խ�࣬������̼��ת����Խ��
(3)���� CO2����������ϩ����ӦΪ 2CO2(g)+6H2(g)![]() C2H4(g)+4H2O(g) ��H��
C2H4(g)+4H2O(g) ��H��
��1L�����ܱ������У�������1molCO2��3molH2���ֱ�ѡ�����ִ�������Ӧ������ͬʱ����CO2ת����[��(CO2)]�淴Ӧ�¶ȵı仯��ͼ2��ʾ��
��ʹ�ô�����ʱ��Ӧ���ʱ�ʹ��ʹ�ô�����죻
�ڸ����¶ȶ�ƽ����ƶ��ж�ƽ�ⳣ���ı仯��
(4)����̼Ԫ�ص������غ�����ɵá�
(1)C(s)��H2(g)��ȼ���ȷֱ���393.5kJmol-1 ��286kJmol-1 ����C(s)+O2(g)=CO2(g)��H=-393.5kJmol-1����H2(g)+![]() O2(g)=H2O(l)��H=-285.8kJmol-1����H2O(l)�TH2O(g)��H2=+44.0kJmol-1�����ݸ�˹���ɣ�����2-��+����2�ɵ�CO2(g)+2H2(g)
O2(g)=H2O(l)��H=-285.8kJmol-1����H2O(l)�TH2O(g)��H2=+44.0kJmol-1�����ݸ�˹���ɣ�����2-��+����2�ɵ�CO2(g)+2H2(g)![]() C(s)+2H2O(g)���ɴ˼�����H=(-286kJmol-1)��2-(-393.5kJmol-1)+(+44.0kJmol-1)��2=-90.5kJmol��1��
C(s)+2H2O(g)���ɴ˼�����H=(-286kJmol-1)��2-(-393.5kJmol-1)+(+44.0kJmol-1)��2=-90.5kJmol��1��
(2)���Է����е��ж���������H-T��S��0���÷�Ӧ��H��0����S��0��Ҫʹ�÷�Ӧ�Է����У�Ӧ���ǵ����Է���
�����������ʵ���Խ�࣬ƽ�������ƶ���������̼��ת����Խ�����Զ�����̼ת����������C�㣬�ʴ�ΪC��
(3)���� CO2����������ϩ����ӦΪ 2CO2(g)+6H2(g)![]() C2H4(g)+4H2O(g) ��H��
C2H4(g)+4H2O(g) ��H��
��1L�����ܱ������У�������1molCO2��3molH2���ֱ�ѡ�����ִ�������Ӧ������ͬʱ����CO2ת����[��(CO2)]�淴Ӧ�¶ȵı仯��ͼ2��ʾ��
����ͼʾ֪��ʹ�ô�����ʱ��Ӧ���ʱ�ʹ��ʹ�ô�����죬��ʹ�ô�����ʱ��Ӧ�Ļ�ܵ���ʹ�ô�����
����ͼʾ��֪ƽ��������¶����ߣ�CO2��ת���ʽ��ͣ�����Ӧ���ȣ�K���¶����߶���С����c��d�����ƽ�ⳣ����С��ϵΪ��Kc��Kd��
(4)0.88gCO2�����ʵ���Ϊ![]() =0.02mol����100mL0.3mol�MLNa2CO3��Һ����0.02mol��CO2���壬������Һ�е�̼Ԫ�ص����ʵ���Ϊ��0.1L��0.3mol/L+0.02mol=0.05mol����̼Ԫ�ص�Ũ��Ϊ0.5mol/L������̼Ԫ�ص������غ㣬��������Һ�У�c(HCO3-)+c(CO32-)+c(H2CO3)=0.5mol/L��
=0.02mol����100mL0.3mol�MLNa2CO3��Һ����0.02mol��CO2���壬������Һ�е�̼Ԫ�ص����ʵ���Ϊ��0.1L��0.3mol/L+0.02mol=0.05mol����̼Ԫ�ص�Ũ��Ϊ0.5mol/L������̼Ԫ�ص������غ㣬��������Һ�У�c(HCO3-)+c(CO32-)+c(H2CO3)=0.5mol/L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�