��Ŀ����

��1��D�Ļ�ѧʽΪ__________________��
��2����Ӧ�۵����ӷ���ʽΪ____________________��
��3��Y��E��һ�������¿ɷ�Ӧ����B��Z������һ������ʵ������ķ�Ӧ��������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
��4��0.1mol/L��X��Һ��0.1mol/L��Y��Һ�������ϣ���Һ��________�ԣ�����ᡱ��������С�������Һ�и�����Ũ�ȴ�С��ϵΪ��_______________________
��5��������0.1mol/L��Y��Һ��c(H+)/c(OH-)=1��10��8������������ȷ����_________
A������Һ��pH=11
B������Һ�е����ʵ������������Ũ��0.1mol/L
C������Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10��22
D��pH=3��������ҺV1 L���0.1mol/L��Y��ҺV2 L��ϣ��������ҺpH=7����:V1>V2
E����pH=11��Y��Һ��ˮϡ��100����pHֵΪ9
��6��������E��һ����������һ������������пɷ�����������ԭ��Ӧ����������������ѹǿ��С��ԭ����2/3����д���÷�Ӧ�Ļ�ѧ����ʽ____________________
��2��3NO2+H2O=2H++2NO3-+NO
��3��4NH3+6NO=5N2+6H2O
��4���c(Cl-)>c(NH4+) >c(H+) >c(OH-)
��5��ACD
��6��3NO==NO2+N2O

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ѧһѡ��3�����ʽṹ�����ʡ���15�֣�
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
��
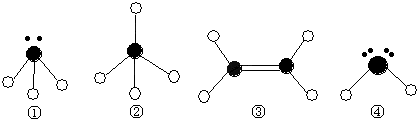
��2��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ

Υ���� ԭ����
��3��Fλ�� �� �������̬ԭ���� ���˶�״̬��
��4��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ�����
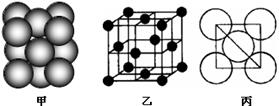
��5����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ��
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ
 ��Υ���� ԭ����
��Υ���� ԭ������Fλ�� �� �������̬ԭ���� ���˶�״̬��
��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ����� ��
����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ������֪�ý�����ԭ�Ӱ뾶Ϊd cm��NA���������ӵ����������������ԭ������ΪM����þ�����ܶ�Ϊ______g��cm��3(����ĸ��ʾ)��

����ѧһѡ��3�����ʽṹ�����ʡ���15�֣�
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
|
AԪ��ԭ�ӵĺ���p����������s����������1 |
|
BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
|
Cԭ�Ӻ�������p���ȫ������� |
|
DԪ�ص������������������IJ�Ϊ4 |
|
E��ǰ�������е縺����С��Ԫ�� |
|
F�����ڱ��ĵ����� |
��1��A��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ����
��
��2��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ
Υ���� ԭ����
��3��Fλ�� �� �������̬ԭ���� ���˶�״̬��
��4��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ�����
��5����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ��