��Ŀ����
����Ŀ��Ϊ�˼����������Ͷ�����̼�Ļ���������Ƿ������һ����̼�������µ�װ�ý���ʵ�顣��ش�
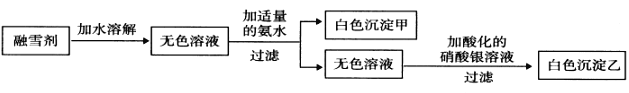
(1)д�����з��ŵ��������ƣ���___________����_____________����_____________��
(2)װ��B���õ��Լ��� _______________��Ŀ����Ϊ��_______________________��
(3)���۲쵽Eװ���г���____________����ʱ��˵�����������һ������һ����̼��
(4)�����������к���һ����̼��Ϊ�˱���������Ӧ��Eװ���ұߵ������ܿڲ�ȡ�Ĵ�ʩ��_____��
(5)Aװ�õ�������___________����Ӧ�Ļ�ѧ����ʽ��___________________��
(6)�����������е�CO��CuO��ȫ��Ӧ����ͨ�������Ϊmg�� D����ng��Eƿ����pg������������CO�������ٷ���Ϊ��_________%�����ȥ��Dװ�ã���������CO�������ٷ���ȷ��Ϊʲô��___________________________________________��
���𰸡�����ƿ ����̨ �ƾ��� ����ʯ��ˮ �����������еĶ�����̼�Ƿ���ȫ���� ��ɫ���ǣ����ɫ������ �鴿���ȼ��������������ϴ��� ��ȥ�����е�CO2 CO2+2NaOH=Na2CO3+H2O ![]() ��ȷ����ΪEƿ���ص�pg������ˮ����������
��ȷ����ΪEƿ���ص�pg������ˮ����������
��������
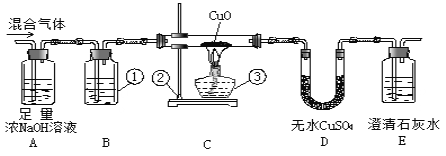
(1)���װ��ͼ��дװ�����ƣ�
(2)���ݼ���һ����̼�ǽ�һ����̼ת��Ϊ������̼���飬���ų����ŷ��������
(3)����һ����̼������ͭ����ͭ�Ͷ�����̼��������̼��ʹ�����ʯ��ˮ����Ƿ�����
(4)����һ����̼���п�ȼ��ȼ�����ɶ�����̼��������̼��������
(5)���ݶ�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ������
(6) ����E�������������������ɵĶ�����̼���������ٸ��ݶ�����̼�����������һ����̼������������һ����̼������������������������ɡ�
(1)����ͼʾ����Ϊ����ƿ����Ϊ����̨����Ϊ�ƾ��ƣ�
(2)��Ϊʵ���Ҽ���һ����̼ͨ����������ת��Ϊ������̼��Ȼ�����ó���ʯ��ˮȥ���������̼�����û��������ԭ�����ж�����̼��Ϊ�˱���������ţ�����Ӧ���ȰѶ�����̼�����꣬Aװ��Ŀ�ľ������ն�����̼��Bװ�����������������ж�����̼�Ƿ�������ȫ������Ӧ���ó���ʯ��ˮ��
(3) ������һ����̼����������ͭ��Ӧ������������ͭ��Ӧ����ˮ����һ����̼������ͭ��Ӧ���ɶ�����̼��������̼����ʹE�г��ֻ�������ֻҪE�б���Ǿ�֤�������������һ����̼��
(4) E�г�������������һ����̼��һ����̼�ж�������ֱ���ŷŵ������У�һ����̼���п�ȼ��ȼ�����ɶ�����̼��������̼����Ϊ�˱���������Ӧ��Eװ���ұߵ������ܿڵ�ȼ���壻
(5)��Ϊʵ���Ҽ���һ����̼ͨ����������ת��Ϊ������̼��Ȼ�����ó���ʯ��ˮȥ���������̼�����û��������ԭ�����ж�����̼��Ϊ�˱���������ţ�����Ӧ���ȰѶ�����̼������ȫ��Aװ��Ŀ�ľ������ն�����̼������Ӧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��
(6)Eƿ����pg��˵�����ɶ�����̼����Ϊpg��������pg������̼��Ҫһ����̼������Ϊx����
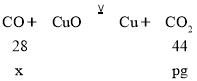
���ݣ�![]() =
=![]() �����x=
�����x=![]() g�����Ի��������CO�������ٷ���Ϊ��
g�����Ի��������CO�������ٷ���Ϊ��![]() ��100%=
��100%=![]() %�����ȥ��Dװ�ã�����������ͭ��Ӧ���ɵ�ˮҲ����Eװ���ڣ�����Ϊ�����ɵĶ�����̼�����������Լ������һ����̼����ƫ��
%�����ȥ��Dװ�ã�����������ͭ��Ӧ���ɵ�ˮҲ����Eװ���ڣ�����Ϊ�����ɵĶ�����̼�����������Լ������һ����̼����ƫ��

����Ŀ������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���![]()
ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
A | �������� | ��ȥ |
B | ��ij��Һ�м��� | ֤����Һ�к� |
C | ��ij��Һ�м���ϡ���ᣬ�ų���ɫ��ζ���壬������ͨ�����ʯ��ˮ��ʯ��ˮ����� | ֤������Һ�д��� |
D | �� | ֤�� |
A.AB.BC.CD.