��Ŀ����
16����Ҫ����д���пո���0.5molH2OԼ��5NA�����ӣ���NA��ʾ����1.204��1024��ˮ���ӵ�����Ϊ36 �ˣ�
��0.5mol Na2CO3��������53�ˣ�����������ˮ�����250mL����Һ�������Һ���ʵ����ʵ���Ũ��Ϊ2mol/L��
�۱�״���£����Ϊ11.2L ��CO2����ԭ�ӵ�������1.5NA������NA��ʾ����
��ͬ��ͬѹ�£�ͬ�����A�����H2�������ֱ���4.8g��0.2g��������A��Ħ������Ϊ48g/mol��
���� ���������ʵ���Ϊˮ��10��������N=nNA����������Ŀ������n=$\frac{N}{{N}_{A}}$����ˮ�����ʵ������ٸ���m=nM����ˮ��������
�ڸ���m=nM����̼���Ƶ�����������c=$\frac{n}{V}$������Һ���ʵ���Ũ�ȣ�
�۸���n=$\frac{V}{{V}_{m}}$���������̼���ʵ���������ԭ�����ʵ���Ϊ������̼��3��������N=nNA���㺬��ԭ��������
��ͬ��ͬѹ�£�ͬ�����A�����H2�����ʵ�����ȣ�����n=$\frac{m}{M}$�����������ʵ������ٸ���M=$\frac{m}{n}$����A�����Ħ��������
��� �⣺���������ʵ���Ϊˮ��10��������������ĿΪ0.5mol��10��NAmol-1=5NA��
1.204��1024��ˮ���ӵ����ʵ���Ϊ$\frac{1.204��1{0}^{24}}{6.02��1{0}^{23}mo{l}^{-1}}$=2mol��ˮ������Ϊ2mol��18g/mol=34g��
�ʴ�Ϊ��5NA��36��
��0.5mol Na2CO3��������0.5mol��106g/mol=53g������������ˮ�����250mL����Һ�������Һ���ʵ����ʵ���Ũ��Ϊ$\frac{0.5mol}{0.25L}$=2mol/L��
�ʴ�Ϊ��53��2��
�۱�״���£����Ϊ11.2L ��CO2�����ʵ���Ϊ$\frac{11.2L}{22.4L/mol}$=0.5mol������ԭ������Ϊ0.5mol��3��NAmol-1=1.5NA��
�ʴ�Ϊ��1.5NA��
���������ʵ���Ϊ$\frac{0.2g}{2g/mol}$=0.1mol��ͬ��ͬѹ�£�ͬ�����A�����H2�����ʵ�����ȣ���A�����Ħ������Ϊ$\frac{4.8g}{0.1mol}$48g/mol��
�ʴ�Ϊ��48g/mol��
���� ���⿼�����ʵ����йؼ��㣬�ѶȲ���ע�����������ʵ���Ϊ���ļ��㣬ּ�ڿ���ѧ���Ի���֪ʶ�Ĺ��̣�

 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�| A�� | pH��7����Һһ���Ǽ���Һ | |
| B�� | pH��ֽ����ˮ��ʪ��һ����Բⶨ������ƫ�� | |
| C�� | pH��ֽӦ������ʪֱ�ӷ������Һ�� | |
| D�� | ����̪��Һ�μӵ�pH��7����Һ�л��� |
| A�� | ��100mL��Ͳȡ5.0mLϡ���� | |
| B�� | ��������ƽȷ��ȡ2.50gNaCl���� | |
| C�� | ʵ������ȡ����ˮ��װ���У��¶ȼ�ˮ����Ӧ����������ƿҺ������ | |
| D�� | ����240mL0.1mol/LNaOH��ҺӦ��ѡ��250mL������ƿ |
| A�� | �����ᡢ��ϡ��Һ��Ӧ���к������ | |
| B�� | ��ͭ˿������β������������á�Hƫ�� | |
| C�� | �ձ�֮����ֽм����Ŀ���DZ����Hƫ�� | |
| D�� | ʵ��ʹ�õĶ�����������Ͳ�����������ձ� |
HUr�����ᣩ+H2O?Ur-����������ӣ�+H3O+
Ur-����Һ��+Na+����Һ��?NaUr���̣�
��һ�ιؽ��������ں��伾�ڽ�ֺ����ָ�Ĺؽڴ���������������ȷ���ǣ�������
| A�� | �������Ա������� | B�� | �����Ƶ��ܽ�����¶����߶����� | ||
| C�� | ��Ӧ��Ϊ���ȷ�Ӧ����Ϊ���ȷ�Ӧ | D�� | �����Ƶ��۵�ܵ� |
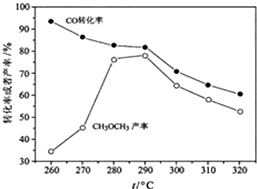 �����ѣ�CH3OCH3������ɫ���壬����Ϊһ��������Դ���ɺϳ��������ΪH2��CO��������CO2��ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ���״��ϳɷ�Ӧ��
�����ѣ�CH3OCH3������ɫ���壬����Ϊһ��������Դ���ɺϳ��������ΪH2��CO��������CO2��ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ���״��ϳɷ�Ӧ��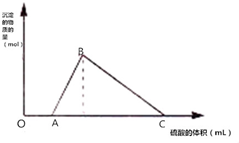 ��M���ƺ����Ļ����Ͷ�뵽һ������ˮ�У�����ȫ���ܽ⣬���ռ�����״�������ΪN�������壬��������Һ����μ���1mol/L H2SO4�����ó��������ʵ��������������Һ������仯��ͼ��ʾ����֪��A��C���Ӧ�ĺ��������ݷֱ���500mL��2500mL����
��M���ƺ����Ļ����Ͷ�뵽һ������ˮ�У�����ȫ���ܽ⣬���ռ�����״�������ΪN�������壬��������Һ����μ���1mol/L H2SO4�����ó��������ʵ��������������Һ������仯��ͼ��ʾ����֪��A��C���Ӧ�ĺ��������ݷֱ���500mL��2500mL����