��Ŀ����
����Ŀ��NiSO46H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����ɵ�Ʒ������������⣬�����У�Cu��Zn��Fe��Cr�����ʣ�Ϊԭ�ϻ�á�������������ͼ��
��ش��������⣺
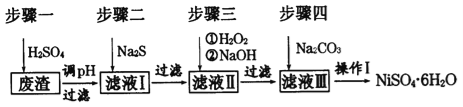
��1����ϡ�����ܽ����ʱ��Ϊ����߽�ȡ���ʿɲ�ȡ�Ĵ�ʩ��_____________(��дһ��)��
��2������Һ�е���������Na2S��Һ��Ŀ���dz�ȥCu2+��Zn2+��д����ȥCu2+�����ӷ���ʽ:_______________��
��3��������������Һ�����Ҫ�ɷ���___________��
��4��ȷ����������Na2CO3��Һ������̼��������ȫ�����ļ�ʵ�鷽����______________��
��5������I��ʵ�鲽������Ϊ(ʵ���п�ѡ�õ��Լ�����Һ������ˮ��pH��ֽ)��
��_______________��
��_______________��
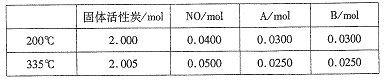
������Ũ������ȴ�ᾧ�����˵�NiSO4��6H2O����
�� �������Ҵ�ϴ��NiSO4��6H2O���岢����
���𰸡���1�����Ȼ�������������Ũ�ȵȣ���2��Cu2++S2-=CuS����
��3��Na2SO4��NiSO4����4���ϲ���Һ����ɫ��
��5���ٹ��ˣ���������ˮϴ����������������м�6mol/L��H2SO4��Һ��ֱ��ǡ����ȫ�ܽ�
��������
�����������1�����ݻ�ѧ��Ӧ���ʵ�Ӱ�����أ���ϡ�����ܽ����ʱ��Ϊ����߽�ȡ���ʿɲ�ȡ�Ĵ�ʩ�м��Ȼ�������������Ũ�ȵȡ�
��2������Na2S��������CuS��������Ӧ�����ӷ���ʽΪCu2++S2-=CuS����
��3����������������Na2S��H2O2��NaOH��ɳ�ȥCu��Zn��Fe��Cr�����ʣ���Һ�к�����������������ΪNa2SO4��NiSO4��
��4����ΪNiSO46H2O��һ����ɫ������ˮ�ľ��壬����ȷ����������Na2CO3��Һ������̼��������ȫ�����ļ�ʵ�鷽�����ϲ���Һ����ɫ��
��5���������ǽ���Һ�е�̼��������ת����NiSO46H2O�����Բ���I��ʵ�鲽������Ϊ�ٹ��ˣ���������ˮϴ����������������м�6mol/L��H2SO4��Һ��ֱ��ǡ����ȫ�ܽ⣻������Ũ������ȴ�ᾧ�����˵�NiSO4��6H2O���壻���������Ҵ�ϴ��NiSO4��6H2O���岢���ɡ�

����Ŀ����1��105 Pa��298 Kʱ����1 mol��̬AB���ӷ������̬Aԭ�Ӻ�Bԭ������Ҫ��������Ϊ����(kJ��mol��1)��������һЩ���ۼ��ļ��ܣ�(��֪���������������ȼ۵ĵ���ۼ�)
���ۼ� | H-H�� | N��N�� | N-H�� |
����(kJ��mol��1) | 436 | 945 | 391 |
��ҵ�ϳɰ��Ļ�ѧ����ʽ��N2��3H2![]() 2NH3��
2NH3��
�Ͽ�1 mol N2�еĻ�ѧ����_____ (����ա��ų���)945 kJ�������γ�2 mol NH3�еĻ�ѧ����_____ (����ա��ų���) ________ kJ������
��298 Kʱ��ȡ1 mol N2��3 mol H2����һ�ܱ������У��ڴ��������½��з�Ӧ�������Ϸų������յ�����ΪQ1����Q1Ϊ____________ kJ��
�����ϱ��е������жϹ�ҵ�ϳɰ��ķ�Ӧ��______(����ȡ����ȡ�)��Ӧ��