��Ŀ����
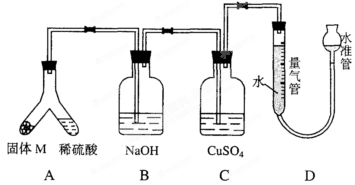
��8�֣�Ϊ�ⶨ��������ĺ�������ȡ0.500g����������������������ȼ�գ�ʹ�����е���ȫ��ת��Ϊ��������ȼ�պ������ͨ����е���ָʾ������Һ�����գ�������֪Ũ�ȵĵ���Һ���еζ���
��1���ζ������з�Ӧ�Ļ�ѧ����ʽ��__________________________��
��2��������ζ��յ�ʱ������VmLŨ��Ϊamol��L��1�ĵ���Һ������ ����������������ļ���ʽ���뻯��Ϊ_____________________________��
����������������ļ���ʽ���뻯��Ϊ_____________________________��
��3��Ϊ�˿��ٲⶨ���������һ��Ũ�ȵĵ���Һʱ��Ҫʹÿ����0.1mL����Һ�൱�������к���0.001%����õ���Һ�����ʵ���Ũ��Ӧ�Ƕ��٣������м�Ҫ������̣�
��1���ζ������з�Ӧ�Ļ�ѧ����ʽ��__________________________��
��2��������ζ��յ�ʱ������VmLŨ��Ϊamol��L��1�ĵ���Һ������
 ����������������ļ���ʽ���뻯��Ϊ_____________________________��
����������������ļ���ʽ���뻯��Ϊ_____________________________����3��Ϊ�˿��ٲⶨ���������һ��Ũ�ȵĵ���Һʱ��Ҫʹÿ����0.1mL����Һ�൱�������к���0.001%����õ���Һ�����ʵ���Ũ��Ӧ�Ƕ��٣������м�Ҫ������̣�
��8�֣�
��1��I2+SO2+2H2O ==H2SO4+2HI ��2�֣�
��2��6.4aV% ��2�֣�
��3��1.56��10-3mol��L��1 ��4�֣�
��1��I2+SO2+2H2O ==H2SO4+2HI ��2�֣�
��2��6.4aV% ��2�֣�
��3��1.56��10-3mol��L��1 ��4�֣�
��

��ϰ��ϵ�д�
�����Ŀ