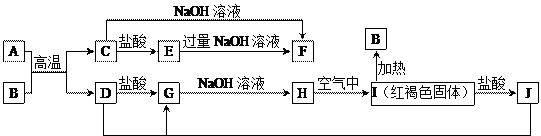��Ŀ����
(l2�֣��±���Ԫ�����ڱ���һ���֣�����������10 ��Ԫ�أ��ش�����
���⡣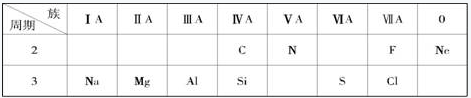
��1���ǽ�������ǿ��Ԫ���� ��
��2��Ne ԭ�ӽṹʾ��ͼΪ ��
��3��C ��N �У�ԭ�Ӱ뾶��С���� ��
��4����ˮ����Ư�����ã����������к��� ���HCl����HClO��)
��5��Ԫ������������Ӧ��ˮ�����У�������ǿ���� ���ѧʽ���������Ե��� ���ѧʽ����
��6��Ԫ�ع�������ﳣ�������� ����һ�ָ����ܵ��ִ�ͨѶ���ϵ�
���ƣ���
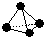
��7����ͼ Ϊij�л�������ģ�ͣ�����
Ϊij�л�������ģ�ͣ����� ������ԭ�Ӵ���
������ԭ�Ӵ��� ̼ԭ�ӣ������л�����̼Ԫ������Ԫ�ص�������m(C):m(H)�� �������ԭ������C-12��H-l)
̼ԭ�ӣ������л�����̼Ԫ������Ԫ�ص�������m(C):m(H)�� �������ԭ������C-12��H-l)
��8��þ�������������ɻ����������Ҫ���ϡ�д����ҵ�ϵ�������Ȼ�þ��ý���þ�Ļ�ѧ����ʽ ��
���⡣

��1���ǽ�������ǿ��Ԫ���� ��
��2��Ne ԭ�ӽṹʾ��ͼΪ ��
��3��C ��N �У�ԭ�Ӱ뾶��С���� ��
��4����ˮ����Ư�����ã����������к��� ���HCl����HClO��)
��5��Ԫ������������Ӧ��ˮ�����У�������ǿ���� ���ѧʽ���������Ե��� ���ѧʽ����
��6��Ԫ�ع�������ﳣ�������� ����һ�ָ����ܵ��ִ�ͨѶ���ϵ�
���ƣ���
��7����ͼ
 Ϊij�л�������ģ�ͣ�����
Ϊij�л�������ģ�ͣ����� ������ԭ�Ӵ���
������ԭ�Ӵ��� ̼ԭ�ӣ������л�����̼Ԫ������Ԫ�ص�������m(C):m(H)�� �������ԭ������C-12��H-l)
̼ԭ�ӣ������л�����̼Ԫ������Ԫ�ص�������m(C):m(H)�� �������ԭ������C-12��H-l) ��8��þ�������������ɻ����������Ҫ���ϡ�д����ҵ�ϵ�������Ȼ�þ��ý���þ�Ļ�ѧ����ʽ ��
(l)F��� (2) 
(3)N (4) HClO (5)NaOH Al(OH)3
(6)���ά (7)6:l (8) MgCl2 Mg+Cl2��
Mg+Cl2��

(3)N (4) HClO (5)NaOH Al(OH)3
(6)���ά (7)6:l (8) MgCl2
 Mg+Cl2��
Mg+Cl2�������������1������Ԫ�����ڱ���֪���ǽ�������ǿ��Ԫ����F��
��2��Ne��ԭ��������10������ԭ�ӽṹʾ��ͼΪ
 .
.��3��ͬ������������ԭ�Ӱ뾶����С�ģ�����C ��N �У�ԭ�Ӱ뾶��С����N��
��4����������ˮ���ɵĴ��������Ư����
��5��������Խǿ������������ˮ����ļ���Խǿ�����Լ�����ǿ�����������ơ������������������������
��6��Ԫ�ع��������������賣����������ά��
��7������ģ�Ϳ�֪���л�������ϩ��CH2=CH2��������̼Ԫ������Ԫ�ص�������m(C):m(H)��24�U4��6:l��
��8��þ�ǻ��õĽ�����Ӧ���õ�ⷨұ�������Թ�ҵ�ϵ�������Ȼ�þ��ý���þ�Ļ�ѧ����ʽ��MgCl2
 Mg+Cl2����
Mg+Cl2��������������ĸ�������������Ԫ�����ڱ��Ľṹ�Լ�Ԫ�������ɣ�����������á�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ����֪����1 mol B��B������167 kJ������������1 mol B��B���ų�942 kJ���������ж���ͬ������B4��B2���ȶ���˳���ǣ�______________________��
����֪����1 mol B��B������167 kJ������������1 mol B��B���ų�942 kJ���������ж���ͬ������B4��B2���ȶ���˳���ǣ�______________________��
 ��3����B��C����Ԫ����ɵĻ�����X��������Ϊ�ӷ��ĵ���ɫҺ�壬X����Ϊ�����η��ӣ��ҷ�����B��C����ԭ���������ﵽ8�����ӵ��ȶ��ṹ��X��ˮ�������γ�һ�ֳ�����Ư�������ʡ���X��ˮ��Ӧ�Ļ�ѧ����ʽ��___________________��
��3����B��C����Ԫ����ɵĻ�����X��������Ϊ�ӷ��ĵ���ɫҺ�壬X����Ϊ�����η��ӣ��ҷ�����B��C����ԭ���������ﵽ8�����ӵ��ȶ��ṹ��X��ˮ�������γ�һ�ֳ�����Ư�������ʡ���X��ˮ��Ӧ�Ļ�ѧ����ʽ��___________________��
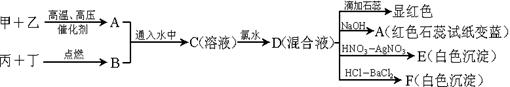
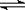 NH4����OH�������ж�NH3����ˮ���γɵ�NH3��H2O�ĺ����ṹ��____ __ (�����) ��
NH4����OH�������ж�NH3����ˮ���γɵ�NH3��H2O�ĺ����ṹ��____ __ (�����) ��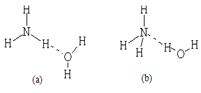
 ������Ӳ�����2����Z��ͬ���ڵ�����Ԫ����ԭ�Ӱ뾶���W�ǵؿ��к������Ľ���Ԫ�أ�L�ĵ��ʾ����۵�ߡ�Ӳ�ȴ���һ����Ҫ�İ뵼����ϡ��û�ѧ����ش��������⣺
������Ӳ�����2����Z��ͬ���ڵ�����Ԫ����ԭ�Ӱ뾶���W�ǵؿ��к������Ľ���Ԫ�أ�L�ĵ��ʾ����۵�ߡ�Ӳ�ȴ���һ����Ҫ�İ뵼����ϡ��û�ѧ����ش��������⣺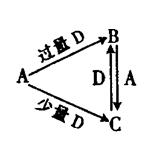
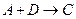 ���Ȼ�ѧ����ʽ��
���Ȼ�ѧ����ʽ��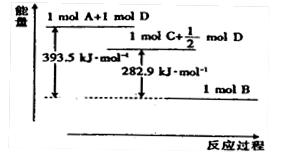
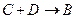 ��
��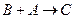 �����ӷ���ʽΪ_________
�����ӷ���ʽΪ_________ _______________________��
_______________________��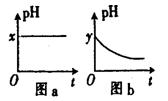
 ���ɵ�ˮ��������������ȥ��
���ɵ�ˮ��������������ȥ��