��Ŀ����
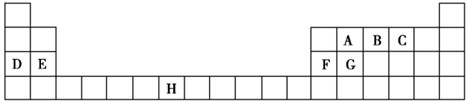
��ͼ��Ԫ�����ڱ���һ���֣����еĢ�~����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش�
 �� ������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | | | | | �� | | �� | |
| �� | �� | �� | �� | �� | | | �� | �� |
| �� | �� | | | | | | �� | |
�Ƶõ���������ǿ���� �ؿ��к������Ľ���Ԫ���� ��
���õ���ʽ��ʾ������γɻ�����Ĺ�����������������������������������
����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ��������������������ǿ���� �������Ե����������� ��
�ɱȽϢڢۢܢ��γɵļ����Ӱ뾶�Ĵ�С���������������� ��
��ijԪ��R����̬�⻯��ΪHXR����R�ڸ��⻯���е���������Ϊ94%��8.5g��HXR�����ڱ�״̬�µ������5.6L����HXR����Է�����Ϊ���� ����HXR�Ļ�ѧʽΪ���� ����
(ÿ��1�֣���10��)
��1��Ar�ṹʾ��ͼ�� ��2��F Al (3) ��4��HClO4 KOH Al(OH)3
��4��HClO4 KOH Al(OH)3
(5)Cl-�� F- ��Na+ ��Mg2+ (6) 34 H2S
���������������Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr��
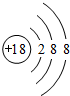
��1������Ԫ����ֻ��Ar������������Ϊ8�����ʲ����ã����ȶ�����ԭ�ӽṹʾ��ͼΪ ��
��
��2���õ���������ǿ����F���ؿ���Ԫ�صĺ����϶����O��Si��Al��Ca�������Ԫ������ΪAl��
��3��������γɵĻ�����ΪMgF2��Ϊ���ӻ���������ʽΪ ������MgF2���γɹ�����
������MgF2���γɹ����� ��
��
��4������Ԫ��������������Ӧ��ˮ������������ǿ��ΪHClO4��������ǿ��ΪKOH�������Ե���������ΪAl��OH��3���ʴ�Ϊ��HClO4��KOH��Al��OH��3��
��5���ڢۢܢ��γɵļ����Ӱ뾶�Ĵ�СΪCl-�� F- ��Na+ ��Mg2+��
��HxR�����ڱ�״���µ������5.6L��˵�����ʵ���Ϊ5.6/22.4=0.25mol������Ϊ��������Ϊ8.5g������Ħ������Ϊ8.5/0.25=34���HxR����Է�������Ϊ34��R�ڸ��⻯���е���������Ϊ94%������R��ԭ����Ϊ34*0.94=31.96=32������R�������HxR�Ļ�ѧʽΪH2S��
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
���������⿼��Ԫ�����ڱ���Ԫ�������ɣ���ϤԪ�������ڱ��е�λ���ǽ����Ĺؼ����ѶȲ���ע�⻯ѧ�����ʹ�������