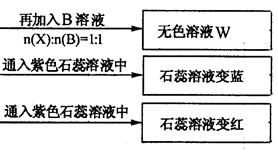
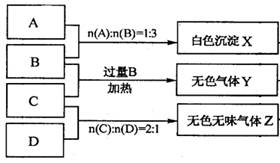
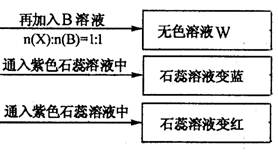
��Ŀ����
(10��)��A��B��C�ֱ��ʾ����10�����ӵ���(����),D��E��F��G�ֱ��ʾ����18�����ӵ���(����),��ش�:
(1) ����A��D��ͬϵ���D���ȴ����� �ֽṹ
(2) B��E������ͬ��Ԫ�أ�B��ˮ��Һ�ʼ��ԣ�E��һ�ֻ��ȼ�ϵijɷ֣���E�ĵ���ʽ
(3) C��F������ͬԪ�أ�д��F��I-��Ӧ�����ӷ�Ӧ����ʽ
(4) G��������Ԫ����ɵ���ԭ�ӷ��ӣ���8.0g NaOH����Һ��ͨ��һ����G���õ�����ҺС�����ɣ��Ƶ���ˮ��7.9g�������ˮ����һ�����е������� ���ѧʽ��
��1�� 2
��2��
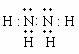
��3��H2O2+2I-+2H+=I2+2H2O
��4�� Na2S

��ϰ��ϵ�д�
�����Ŀ