��Ŀ����
4��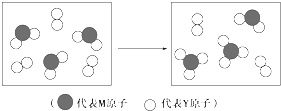 Ԫ��X��Y��Z��M��NΪ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��Mԭ�ӵ����������������������֮��Ϊ3��4��Mԭ�ӵ���������Yԭ�ӵ�2����N-��Z+��X+�İ뾶��С��������XN�ڳ�����Ϊ���壬�ݴ˻ش��������⣺
Ԫ��X��Y��Z��M��NΪ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��Mԭ�ӵ����������������������֮��Ϊ3��4��Mԭ�ӵ���������Yԭ�ӵ�2����N-��Z+��X+�İ뾶��С��������XN�ڳ�����Ϊ���壬�ݴ˻ش��������⣺��1���õ���ʽ��ʾZ��N�γɻ�����Ĺ���
 ��
����2��X��Y�ɷֱ��γ�10���Ӻ�18���ӵķ��ӣ�д����18���ӷ���ת����10���ӷ��ӵĻ�ѧ����ʽ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2�����ֽⷴӦ����
��3����ͼ��ʾ������Ԫ����ɵ��������������һ�������µ��ܱ������г�ַ�Ӧǰ���ת����ϵ����д����ת�����̵Ļ�ѧ����ʽ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��4��A��B��Ϊ����������Ԫ���е�����Ԫ����ɵ�ǿ����ʣ������Ԫ�ص�ԭ�Ӹ���֮��Ϊ1��1��1�����ڸ��Ե�ˮ��Һ�У�A������ˮ�ĵ��룬B�ܴٽ�ˮ�ĵ��룬��A�Ļ�ѧʽΪNaOH��B�Ļ�ѧʽΪNaHS��A��B֮�䷴Ӧ�����ӷ���ʽΪOH-+HS-=H2O+S2-��A�д��ڵĻ�ѧ������Ϊ�����Ӽ������ۼ���
���� Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ��������������YԪ��ԭ������������������������֮��Ϊ3��4������������ֻ��Ϊ6����ԭ������Ϊ8��YΪOԪ�أ�Mԭ�ӵ���������Yԭ�ӵ�2������MΪSԪ�أ����N-��Z+��X+�İ뾶��С��������XN�ڳ�����Ϊ���壬����NΪClԪ�أ�ZΪNaԪ�أ�XΪ��Ԫ�أ��ݴ˽��
��� �⣺Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ��������������YԪ��ԭ������������������������֮��Ϊ3��4������������ֻ��Ϊ6����ԭ������Ϊ8��YΪOԪ�أ�Mԭ�ӵ���������Yԭ�ӵ�2������MΪSԪ�أ����N-��Z+��X+�İ뾶��С��������XN�ڳ�����Ϊ���壬����NΪClԪ�أ�ZΪNaԪ�أ�XΪ��Ԫ�أ�
��1��Z��N�γɵĻ�����Ļ�ѧʽΪNaCl���õ���ʽ��ʾ���γɹ���Ϊ
�ʴ�Ϊ�� ��
��
��2��H��O�ɷֱ��γ�10���Ӻ�18���ӵķ��ӣ��ֱ�ΪH2O��H2O2��H2O2�ڶ������������������·ֽ�����ˮ����������Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��3����ͼ��֪����ת������ΪSO2��O2��Ӧ������������Ӧ��ѧ����ʽΪ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
�ʴ�Ϊ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��4��A��B��Ϊ����������Ԫ���е�����Ԫ����ɵ�ǿ����ʣ������Ԫ�ص�ԭ�Ӹ���֮��Ϊ1��1��1�����ڸ��Ե�ˮ��Һ�У�A������ˮ�ĵ��룬˵��A�����B�ܴٽ�ˮ�ĵ��룬˵��B�Ǻ��������ӵ��Σ�AΪNaOH��BΪNaHS�ȣ�A��B֮�䷴Ӧ�����ӷ���ʽΪ��OH-+HS-=H2O+S2-��A�������Ӻ�����������֮��������Ӽ���Oԭ�Ӻ�Hԭ��֮����ڹ��ۼ�������A�к������Ӽ����ۼ���
�ʴ�Ϊ��NaOH��NaHS��OH-+HS-=H2O+S2-�����Ӽ������ۼ���
���� ���⿼��ṹ�������ʹ�ϵӦ�ã��ѶȲ����ݺ�������Ų������ƶ�Ԫ���ǽ���ؼ������ضԻ���֪ʶ�Ĺ��̣�

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�| A�� | ���º��ݣ�����1����1mol N2+3mol H2������2����2mol NH3 | |
| B�� | ���º�ѹ������1����1mol N2+3mol H2������2����2mol NH3 | |
| C�� | ���º��ݣ�����1����1mol N2+3mol H2������2����3mol NH3 | |
| D�� | ���º�ѹ������1����1mol N2+3mol H2������2����3mol NH3 |
| A�� | ��ⱥ��ʳ��ˮ�����ӷ���ʽ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2�� | |
| B�� | �����KI��Һ�еμ�ϡ������Һ������4I-+O2+4H+=2H2O+2I2 | |
| C�� | ���������ܲ�����������Һ�п��ܴ��ڴ�����K+��Ba2+��AlO2-��Cl- | |
| D�� | HCO3-ˮ������ӷ���ʽ��HCO3-+H2O=CO32-+H3O+ |
| A�� | ���������Գ�ȥ�������е�����̼�������� | |
| B�� | ����ʱ�����ȵ��������г��ֽ϶�������ʱֹͣ���� | |
| C�� | �÷�Һ©������Һ��ʱ���ȷų��²�Һ����ٷų��ϲ�Һ�� | |
| D�� | ����ʱ���ò���������©���ڵĻ��Һ�Լӿ�����ٶ� |