��Ŀ����
����Ŀ��ʵ��������NaOH��������1.0 mol��L��1��NaOH��Һ240 mL��
(1)������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ������ڼ��㡡���ܽ⡡��ҡ�ȡ���ת�ơ���ϴ�ӡ��߶��ݡ�����ȴ����ҡ��
����ȷ�IJ���˳��Ϊ�ڢ٢�__________________�ߢܡ���ʵ������õ�����������ƽ��ҩ�ס����������ձ���__________________________��
(2)ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ��ʾ��
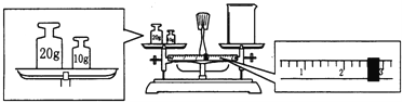
�ձ���ʵ������Ϊ________g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�________g NaOH��
(3)ʹ������ƿǰ������е�һ��������_________________��
(4)�����ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���___________��
A��ת����Һʱ������������������ƿ����
B������ʱ���ӿ̶���
C��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
D�����ݺ�����ƿ��������תҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶���
���𰸡���ݢޢ�250 mL����ƿ����ͷ�ι�27.410.0��©BC
��������
(1)����һ�����ʵ���Ũ����Һһ�㲽��:���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵ�����ȷ��˳��Ϊ�ڢ٢ۢ�ݢޢ�ߢܣ��õ�������:��ƽ��ҩ�ס����������ձ�����ͷ�ιܡ�250mL����ƿ���������ȱ�ٵ�����Ϊ: 250mL ����ƿ; �ͽ�ͷ�ι��� �𰸣���ݢޢ� 250 mL����ƿ����ͷ�ι� ��
(2)����ͼ����֪��:����Ϊ30g,����Ϊ2.6g,��Ϊ����ƽ��������ʱ���������룬��ʵ�����ʺ�����ŷ��ˣ������ձ���ʵ������Ϊ30g -2.6g= 27.4 g;Ҫ��NaOH��������1.0 mol��L��1��NaOH��Һ240 mL��Ӧѡ��250mL ����ƿ,��Ҫ������������m=0.25mol![]() 40g/mol=10g����ˣ�������ȷ����:27.4�� 10.0��
40g/mol=10g����ˣ�������ȷ����:27.4�� 10.0��
(3)����ƿ���л���,ʹ�ù�������Ҫ���µߵ�ҡ��,����ʹ��ǰӦ����Ƿ�©ˮ; ��ˣ�������ȷ����:��©;
(4) A��ת����Һʱ������������������ƿ���棬�������ʵ�����С��������ҺŨ��ƫ�͡���A����B.����ʱ���ӿ̶���ˮ�������С�ˣ�������ҺŨ��ƫ�ߣ���B�������⣻C��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݵ�����Һ�����С����ҺŨ��ƫ�ߣ���C�������⣻D�����ݺ�����ƿ������ҡ��,���ú�,Һ����ڿ̶���,�ټ�ˮ���̶���,������Һ���ƫ��,��ҺŨ��ƫ��,�ʲ�ѡ���𰸣�BC��

����Ŀ��ͭ���仯���������ǵ��ճ����������Ź㷺����;���ش���������:
��1��ͭ��ͭ�ε���ɫ��ӦΪ��ɫ�������й�ԭ��������������ȷ����_____(����ĸ)��
a.���Ӵӻ�̬ԾǨ���ϸߵļ���̬ b.���Ӵӽϸߵļ���̬ԾǨ����̬
c.��ɫ��Ӧ�Ĺ����������չ��� d.��ɫ��Ӧ�Ĺ������ڷ������
��2����̬Cuԭ���У��������ռ�ݵ�����ܲ������_____�����������Ų�ʽ��δ�ɶԵ�����Ϊ______����Cu��Ag������IB�壬�۵�:Cu____Ag(����>������<��)��
��3��[Cu(NH3)4]SO4�������ӵ����幹����_________������ԭ�ӵĹ���ӻ�����Ϊ__________��[Cu(NH3)4]SO4��Cu2+��NH3֮���γɵĻ�ѧ����Ϊ________________��
��4����Cu���������������Ҵ�������ȩ����ȩ�������������ı�ֵΪ___________��
��5���⡢ͭ����Ԫ�صĵ縺�����:
Ԫ�� | I | Cu |
�縺�� | 2.5 | 1.9 |
CuI����_______(��������������������)��������
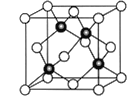
��6��Cu��Cl�γ�ij�ֻ�����ľ�����ͼ��ʾ���þ�����ܶ�Ϊ��g��cm-3�������߳�Ϊacm�����ӵ�����Ϊ__________(�ú��ѡ�a�Ĵ���ʽ��ʾ)��