��Ŀ����
���л�ѧ���������ȷ���ǣ� ��
| A������ı�ȼ���ȡ�H="-890.3" kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g)=CO2(g)+2H2O(l)��H="-890.3" kJ��mol-1 |
B����ϩ�ļ���ʽ��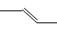 |
C��һ�������£�ij�ܱ������г���2 mol SO2(g)��1 mol O2(g)���ﵽƽ��ų�Q kJ��������������µ��Ȼ�ѧ����ʽΪ��2SO2(g)+O2(g) 2SO3(g )��H=" -Q" kJ��mol-1 2SO3(g )��H=" -Q" kJ��mol-1 |
| D����ϩ�Ľṹ��ʽ��CH2CH2 |
A
�������������A������ı�ȼ���ȡ�H="-890.3" kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H="-890.3" kJ��mol-1����ȷ��B�� Ϊ2����ϩ�ļ���ʽ������C��2SO2+O2
Ϊ2����ϩ�ļ���ʽ������C��2SO2+O2 2SO3Ϊ���淴Ӧ�����淴Ӧ���ܽ��е��ף�һ�������£�ij�ܱ������г���2 mol SO2(g)��1 mol O2(g)���ﵽƽ��ų�Q kJ��������������µ��Ȼ�ѧ����ʽ���ʱ��H�� -Q kJ��mol-1������D����ϩ�Ľṹ��ʽӦΪCH2=CH2������
2SO3Ϊ���淴Ӧ�����淴Ӧ���ܽ��е��ף�һ�������£�ij�ܱ������г���2 mol SO2(g)��1 mol O2(g)���ﵽƽ��ų�Q kJ��������������µ��Ȼ�ѧ����ʽ���ʱ��H�� -Q kJ��mol-1������D����ϩ�Ľṹ��ʽӦΪCH2=CH2������
���㣺���黯ѧ���

��NAΪ����ӵ���������ֵ������˵����ȷ����
| A��1 L 0.1mol?L��1Na2CO3��Һ����0.1NA��CO32- |
| B�����³�ѹ�£�22gCO2�й���2NA�����õ��Ӷ� |
| C��0.1mol Na2O2��ˮ��ȫ��Ӧ��ת�Ƶĵ�����Ϊ0.2NA |
| D��2.8gN2��CO��C2H4��ɵĻ��������ռ�е����ԼΪ2.24L |
��֪�����ӵ����������ʵ�Ħ��������1Ħ�����ʵ�������������������в���ȫ������������ֵ����( )
| A���������ʷ��ӵĴ�С������ | B��Һ̬���ʷ��ӵĴ�С������ |
| C���������ʷ��ӵĴ�С������ | D���������ʷ��ӵ����� |
��һ�����¶ȡ�ѹǿ�£���100 mLCH4��Ar�Ļ��������ͨ��400 mL O2����ȼʹ����ȫ��Ӧ���������ͬ�����µõ���������460 mL����Ӧǰ���������CH4��Ar�����ʵ���֮��Ϊ (�� ��)
| A��1��4 | B��1��3 | C��1��2 | D��1��1 |
��NA��ʾ�����ӵ�����,���й���0��2 mol��L-1K2SO4��Һ����ȷ˵���� �� ��
| A��1 L��Һ��K+Ũ����0��4 mol��L-1 |
| B����Һ�к���0��4NA��K+ |
| C��1 L��Һ������K+��SO42-����Ϊ0��3NA |
| D��2 L��Һ��SO42-Ũ����0��4 mol��L-1 |
ij�������ռ���Ʒ����Na2CO3 3.8%�������ٷֺ�������H2O 5.8%��NaHCO3 0.004%��ȡm g��Ʒ����40 m L 2 mol/L���������2 mol/L Na OH��Һ�к�ʣ����ᣬ��������ɵø���Ĺ��������Ϊ�� ��
| A��4.68 g | B��5.58 g | C��2.34 g | D��3.34 g |
��һ�ֻ���ɫ����X�������Ϊ20��������ȫ�ֽ������10������������20����������ͬ��ͬѹ�²ⶨ�����ж�XΪ��ԭ�ӷ������ݵĶ�����( )
�������غ㶨�� �������غ㶨�� ���������ԭ�� �ܰ����ӵ�����
| A���٢� | B���٢� | C���ڢ� | D���ڢ� |
NAΪ�����ӵ�����������˵������ȷ���ǣ� ��
| A����״���£�22.4LH2O������ԭ����Ϊ3NA | |
| B��1molOH���к�������ĿΪ9NA | |
| C����״���£�11.2LN2��O2�Ļ������������ԭ����ΪNA | D��1 mol/L MgSO4��Һ������SO42-��ĿΪNA |
��0.5molNa2SO4�к���Na������ĿԼΪ
| A��3.01��1023 | B��6.02��1023 | C��0.5 | D��1 |