��Ŀ����
����Ŀ����ʽ̼��ͭ�㷺�������������ϡ��̻�ɱ���������������ͭ�κ���ӫ��ۼ�����ȣ�Ҳ�������Ӵ�����ɱ�����ȡ�ij��ѧ��ȤС����ʵ��������Na2CO3��10H2O��CuSO4��5H2O��Ӧ�Ʊ��������ʽ̼��ͭ��ʵ�鲽�����£�

(1)д���Ʊ���ʽ̼��ͭ�Ļ�ѧ����ʽ_________________��
(2)���β��ֱ���ϸNa2CO3��10H2O��CuCO4��5H2O��Ŀ����___________________��
(3)��������ɫ�����Ƿ�ϴ�Ӹɾ���ʵ�������__________________________��
(4)��ʽ̼��ͭ���Կ���Cu(OH)2��CuCO3������ȤС��ͬѧѡ������ʵ��������֤�����к���Cu(OH)2��CuCO3��

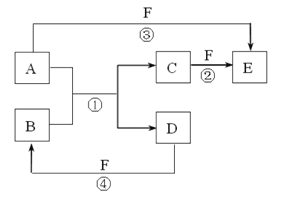
�ٸ�װ������˳��Ϊ____________________________��
��װ��B���Լ���������________��װ��C���Լ���������________��
��֤������CuCO3��ʵ��������_________________��
֤������Cu(OH)2��ʵ��������______________________��
���𰸡�(1)2CuSO4��2Na2CO3��H2O===Cu2(OH)2CO3����2Na2SO4��CO2��
(2)����Ӧ��Ӵ������ʹ��Ӧ���ֻ�ϲ���Ӧ��
(3)ȡ���һ��ϴ��Һ�������μ�BaCI2��Һ����û�а�ɫ�����������������ϴ�Ӹɾ�
(4)��ACB �ڳ���ʯ��ˮ ��ˮ����ͭ��ĩ��װ��B�г���ʯ��ˮ����� װ��C�а�ɫ��ĩ��Ϊ��ɫ
��������(1)�Ʊ���ʽ̼��ͭ��������CuSO4��Na2CO3��ˮ����ٽ������ʡ�
(2)���в��ֱ���ϸNa2CO3��10H2O��CuSO4��5H2O��Ϊ������Ӧ��ĽӴ�������Ƿ�Ӧ���ֻ�Ϸ�Ӧ��
(3)������ɫ����ϴ�Ӹɾ��������ϴ��Һ�в���SO![]() ���μ�BaCI2��Һ��û�а�ɫ����������
���μ�BaCI2��Һ��û�а�ɫ����������
(4)������ˮ����ͭ�ɼ�������Ⱥ�IJ����д��ڵ�H2O���Ӷ�֤������Cu(OH)2�����ó���ʯ��ˮ�ɼ�������Ⱥ�IJ����к���CO2���Ӷ�֤������CuCO3�����Ǽ���H2O�����ڼ���CO2֮ǰ��

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�