��Ŀ����
����Ŀ��(1)��֪2 mol����ȼ������Һ̬ˮʱ�ų�572 kJ����������Ӧ����ʽ��2H2(g)��O2(g)===2H2O(l)����ش��������⣺
�ٸ÷�Ӧ�������������ܺ�________(��������������С��������������)��Ӧ�������ܺ͡�
����2 mol������ȫȼ������ˮ��������ų�������_____(��������������С��������������)572 kJ��
(2) 2.3g�л���C2H6O��һ������������ϵ�ȼ,ǡ����ȫȼ��,����CO2��Һ̬ˮ�����ų�68.35kJ����,��÷�Ӧ���Ȼ�ѧ����ʽ��________________________________��
(3) FeS2���ղ�����SO2�����������ᡣ��֪25 ����101 kPaʱ��
2SO2(g)��O2(g) ![]() 2SO3(g)����H1����197 kJ��mol��1
2SO3(g)����H1����197 kJ��mol��1
H2O(g)===H2O(l)����H2����44 kJ��mol��1
2SO2(g)��O2(g)��2H2O(g)===2H2SO4(l)����H3����545 kJ��mol��1
��SO3(g)��H2O(l)��Ӧ����H2SO4(l)���Ȼ�ѧ����ʽ��_________________________________��
���𰸡� С�� С�� C2H6O��l��+3O2��g��=2CO2��g��+3H2O(l)��H=-1367kJ/mol SO3��g����H2O��l��=H2SO4 ��l����H����152 kJ/mol
��������(1)������ȼ������Һ̬ˮʱ���ȣ���Ӧ�������������������������ʴ�Ϊ��С�ڣ�
��Һ̬ˮ��Ϊˮ���������ȵĹ��̣�2mol����ȼ������Һ̬ˮʱ�ų�572kJ������������̬ˮʱ�ų�������С��572kJ���ʴ�Ϊ��С����
(2) 2.3g�л���C2H6O�����ʵ���Ϊ![]() =0.05mol����һ������������ϵ�ȼ��ǡ����ȫȼ��������CO2��Һ̬ˮ�����ų�68.35kJ��������1molC2H6O��ȫȼ�շų�������Ϊ68.35kJ��
=0.05mol����һ������������ϵ�ȼ��ǡ����ȫȼ��������CO2��Һ̬ˮ�����ų�68.35kJ��������1molC2H6O��ȫȼ�շų�������Ϊ68.35kJ��![]() =1367kJ����÷�Ӧ���Ȼ�ѧ����ʽΪC2H6O(l)+3O2(g)=2CO2(g)+3H2O(l)��H=-1367kJ/mol���ʴ�Ϊ��C2H6O(l)+3O2(g)=2CO2(g)+3H2O(l)��H=-1367kJ/mol��
=1367kJ����÷�Ӧ���Ȼ�ѧ����ʽΪC2H6O(l)+3O2(g)=2CO2(g)+3H2O(l)��H=-1367kJ/mol���ʴ�Ϊ��C2H6O(l)+3O2(g)=2CO2(g)+3H2O(l)��H=-1367kJ/mol��
(3)2SO2(g)+O2(g)![]() 2SO3(g)��H1=һ197kJ/mol ����2H2O(g)=2H2O(1)��H2=-44kJ/mol ����2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l)��H3=һ545kJ/mol�������ø�˹���ɣ�(��-��-��)��
2SO3(g)��H1=һ197kJ/mol ����2H2O(g)=2H2O(1)��H2=-44kJ/mol ����2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l)��H3=һ545kJ/mol�������ø�˹���ɣ�(��-��-��)��![]() ��SO3 (g)+H2O(l)=H2SO4(l)��H=-152kJ/mol���ʴ�Ϊ��SO3(g)+H2O(l)=H2SO4(l)��H3=-152kJ/mol��
��SO3 (g)+H2O(l)=H2SO4(l)��H=-152kJ/mol���ʴ�Ϊ��SO3(g)+H2O(l)=H2SO4(l)��H3=-152kJ/mol��

����Ŀ����������������Ҫ��Fe2O3��SiO2��Al2O3�����ʣ��Ʊ��������Ĺ�������������
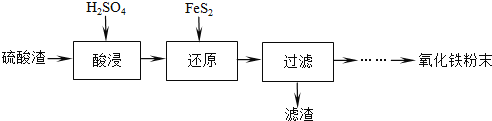
�±��г�����ظ�ʵ�������½������������������������pH��
������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 |
��ʼ���� | 2.7 | 3.8 | 7.5 |
��ȫ���� | 3.2 | 5.2 | 9.7 |
��1���������ʱ����Ҫ�ʵ���������Ŀ�ij���������Ľ���������������_______����������������Ҫ�ⶨ��Һ��Fe3+�ĺ�������ԭ����____________��
��2������ԭ���ǽ�Fe3+ת��ΪFe2+��ͬʱFeS2������ΪSO42-���÷�Ӧ�����ӷ���Ϊ________��
��3����������Ҫ�ɷ���FeS2��_______(�ѧʽ)��
��4�����˺����Һ�к���Fe3+������Fe3+��ԭ����________(�����ӷ���ʽ��ʾ����
��5��������á����ˡ������Һ�Ʊ�Fe2O3��ʵ�鷽����___________��ʵ���б���ʹ�õ��Լ��У�5% ��H2O2��Һ��0.5mol/LNaOH��Һ����
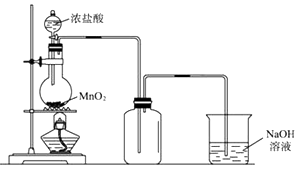
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��
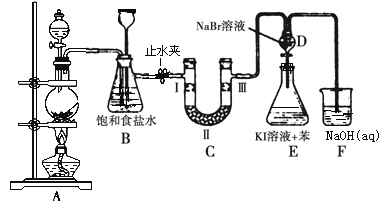
��1��ʵ�����������Ļ�ѧ����ʽΪ____________________________��
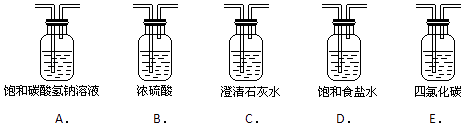
��2��װ��B�б���ʳ��ˮ��������______________����д��װ��B����һ������_________________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ� Ϊ��C�������η������ʵ������________(����)��
��� | a | b | c | d |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ���Ӧһ��ʱ���������װ��D��������Һ����װ��E�У����۲쵽��������____________________��������_______(������������������)˵����ķǽ�����ǿ�ڵ⣬ԭ����_______________��
��5�����������װ��F�пɸ���������Na2SO3��Һ�������ȣ���д����Ӧ�����ӷ�Ӧ����ʽ��__________________��