��Ŀ����
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�顣
������ʽ��ȷ����
��1�����л���A�����������г��ȼ�գ�ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L����״���£�����������и�Ԫ�ص�ԭ�Ӹ������� ��
��2�������Dzⶨ�л����������Է�������Ϊ46��������ʵķ���ʽ�� ��
��3�����ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ ��
���ṹʽ��ȷ����
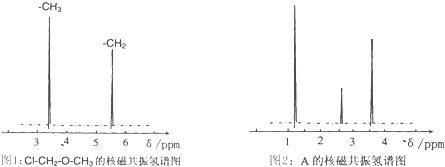
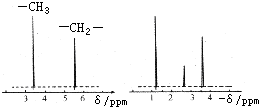
��4���˴Ź�����ԭ�ӹ����ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ���źţ�����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ��ѣ�Cl�DCH2�DO�DCH3����������ԭ�ӣ���ͼ1�������ⶨ���л���A�ĺ˴Ź�������ʾ��ͼ��ͼ����A�Ľṹ��ʽΪ ��

ͼ1 ![]() ͼ2
ͼ2 ![]()
������ʵ�顿
��5��A��һ����������ˮ������B��B�ɺϳɰ�װ����C����д��Bת��ΪC�Ļ�ѧ��Ӧ����ʽ�� ��
��6�����������е��˶�Ա����Ť��ʱ����ҽ�漴�������飨�е�Ϊ12.27�棩�����˲�λ���оֲ��䶳����������Bѡ����ʵķ����Ʊ������飬Ҫ��ԭ��������Ϊ100%����д���Ʊ���Ӧ����ʽ�� ��
��7��A��ͨ����ʳ��һ���������Ƶã�����ʳ�Ƶõ�A��һ���¶����ܱմ��棬��Ϊ����һϵ�еĻ�ѧ�仯����ø����㡣��д�����һ����Ӧ�Ļ�ѧ����ʽ��
��
��1��n(C): n(H): n(O)= 2:6:1
��2��C2H6O
��3��CH3CH2OH CH3O-CH3
��4��CH3CH2OH
��5��nCH2=CH2![]()
![]()
��6��nCH2=CH2 + HCl![]() CH3CH2 Cl
CH3CH2 Cl
��7��![]()
![]()
![]()

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�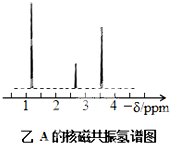 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮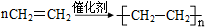
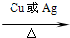 2CH3CHO+2H2O
2CH3CHO+2H2O
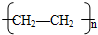 ��
�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O��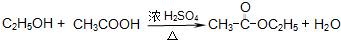
 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮