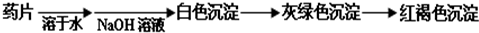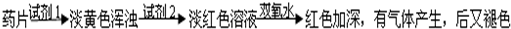题目内容
某学校同学进行乙醇的化学性质实验探究学习,以下为他们的学习过程。
(一)结构分析
(1)写出乙醇的结构式 ;官能团的电子式 。
(二)性质预测
(2)对比乙醇和乙烷的结构,经过讨论,同学们认为乙醇分子中氧原子吸引电子能力较强,预测在一定条件下分子中 键(填写具体共价键)容易发生断裂。为此,他们查阅了相关资料,获得了以下乙醇性质的部分事实。
|
下列关于乙醇结构和性质的分析、理解错误的是
A.-OH对-C2H5的影响使乙醇与钠的反应比水与钠的反应速率慢ks5u
B. 乙醇在浓硫酸催化下,170℃时脱水生成乙烯的反应为消去反应
C. 乙醇的核磁共振氢谱图上有3个吸收峰,其强度之比为3︰2︰1,与钠反应的是
吸收强度最小的氢原子
 D. 乙醇与甲醚互为官能团异构体
D. 乙醇与甲醚互为官能团异构体
(三)设计方案、进行实验
甲同学用4mL 95%的乙醇、8mL90%浓硫酸、
6g溴化钠研究乙醇转化为溴乙烷的反应。右图
是他设计的实验装置图(已省略部分夹持仪器)。
请回答有关问题。
(3)预计实验时装置Ⅰ主要发生两个反应,写出反应②的化学方程式。
![]() ①2NaBr + H2SO4 2HBr + Na2SO4,
①2NaBr + H2SO4 2HBr + Na2SO4,
②
(4)实验过程中,观察到反应后期烧瓶内液体颜色变棕黑,U形管右边与大气相通的导管口产生大量有刺激性气味的白雾,U形管内有少量淡黄色液体,该液体的有机成分是 。若要获得纯净的溴乙烷,方法是:实验结束后 。
(四)反思与改进
(5)乙同学认为:实验所用浓硫酸必须进行稀释,目的是 (填字母),稀释后的浓硫酸应放在 (填实验仪器名称)中。
A.减少HBr的挥发 B.防止浓硫酸分解产生SO2
C.减少副产物乙烯和乙醚的生成 D.减少Br2的生成
(6)丙同学提出应该对实验装置进行改进,请为两部分装置选择正确的措施:
A.不作改变 B.保留酒精灯加热,增加温度计且温度计水银球插入反应液中
C.水浴加热 D.冰水混合物冷却
装置Ⅰ ;装置Ⅱ 。请你再提出一条改进措施 。
(1) ![]() ,
,![]() (各1分,共2分)
(各1分,共2分)
(2)C—O键与O—H键,A(各2分,共4分)
(3) ![]() (2分)
(2分)
(4) 溴乙烷、乙醇和乙醚(1分),把U形管内的液体倒入装有NaOH溶液的烧杯中,充分振荡后转移至分液漏斗内,分液取出下层液体(3分)。
(5)ACD(2分),分液漏斗(1分)
(6)C,D, 装置I把短导管换成长导管或增加冷凝管,装置Ⅱ与大气相通的导管接装有碱石灰的球形干燥管。 (各1分,共3分)

 提分百分百检测卷单元期末测试卷系列答案
提分百分百检测卷单元期末测试卷系列答案 小学期末标准试卷系列答案
小学期末标准试卷系列答案(12分)补铁药物“速力菲”说明书部分内容摘录:
【规格】每片含琥珀酸亚铁0.1g
【组成与性状】内含Fe2+34.0%~36.0%的无水碱式盐,为薄膜糖衣片
【适应症】用于缺铁性贫血症预防及治疗
【用量用法】成人预防量0.1g/日,治疗量0.2g~0.4g/日;小儿预防量30~60mg/日,治疗量0.1g~0.3g/日
【储藏】在避光、密封、阴凉处保存
【药物相互作用】与维生素C同服,可增加本品吸收;该药片在水和乙醇中溶解度不大。
现某学校化学实验小组,为了检测“速力菲”药片中亚铁元素的存在,设计了如下几组实验:
Ⅰ、理论设计方案: 甲组同学按照设计的方案完成实验,但遗憾的是他们没有得到预期的实验结果,
(1)甲组实验失败的可能原因:________________________________________________。
乙组同学认真思考了甲组实验失败的原因,模拟药片服用后在人体中溶解的变化过程,重新设计并完成下列实验:
(2)若试剂1为盐酸;则试剂2为________________________________。
丙组同学对乙组实验中最后红色褪去的现象产生了研究兴趣,探讨褪色的原因,根据微粒的组成,他们认为有两种可能的原因:
[Fe(SCN)]2+络离子中的三价铁被还原为亚铁
② 。
(3)请你对其中一种可能进行实验验证:________________________________
实验方案(用文字述):________________________________________________,
根据预期现象判断结论:________________________________________________________。
Ⅱ、(4)称量“速力菲”1.0 g,将其全部溶于稀硫酸中,配制成100.00 mL溶液,取出20.00 mL,用0.01000 mol/L的KMnO4溶液滴定。三次操作读数如下:
| 序号 | V(KMnO4)初 | V(KMnO4)终 | V(KMnO4) |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |
计算:该补血药中含Fe2+的质量分数________________(保留小数点后二位小数)。
(12分)补铁药物“速力菲”说明书部分内容摘录:
【规格】每片含琥珀酸亚铁0.1g
【组成与性状】内含Fe2+34.0%~36.0%的无水碱式盐,为薄膜糖衣片
【适应症】用于缺铁性贫血症预防及治疗
【用量用法】成人预防量0.1g/日,治疗量0.2g~0.4g/日;小儿预防量30~60mg/日,治疗量0.1g~0.3g/日
【储藏】在避光、密封、阴凉处保存
【药物相互作用】与维生素C同服,可增加本品吸收;该药片在水和乙醇中溶解度不大。
现某学校化学实验小组,为了检测“速力菲”药片中亚铁元素的存在,设计了如下几组实验:
Ⅰ、理论设计方案: 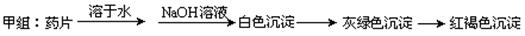 甲组同学按照设计的方案完成实验,但遗憾的是他们没有得到预期的实验结果,
甲组同学按照设计的方案完成实验,但遗憾的是他们没有得到预期的实验结果,
(1)甲组实验失败的可能原因:________________________________________________ 。
乙组同学认真思考了甲组实验失败的原因,模拟药片服用后在人体中溶解的变化过程,重新设计并完成下列实验:
(2)若试剂1为盐酸;则试剂2为________________________________。
丙组同学对乙组实验中最后红色褪去的现象产生了研究兴趣,探讨褪色的原因,根据微粒的组成,他们认为有两种可能的原因:
[Fe(SCN)]2+络离子中的三价铁被还原为亚铁
② 。
(3)请你对其中一种可能 进行实验验证:________________________________
进行实验验证:________________________________
实验方案(用文字述):________________________________________________ ,
根据预期现象判断结论:________________________________________________________ 。
Ⅱ、(4)称量“速力菲”1.0 g,将其全部溶于稀硫酸中,配制成100.00 mL溶液,取出20.00 mL,用0.01000 mol/L的KMnO4溶液滴定。三次操作读数如下:
| 序号 | V(KMnO4)初 | V(KMnO4)终 | V(KMnO4) |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |
(12分)补铁药物“速力菲”说明书部分内容摘录:
【规格】每片含琥珀酸亚铁0.1g
【组成与性状】内含Fe2+34.0%~36.0%的无水碱式盐,为薄膜糖衣片
【适应症】用于缺铁性贫血症预防及治疗
【用量用法】成人预防量0.1g/日,治疗量0.2g~0.4g/日;小儿预防量30~60mg/日,治疗量0.1g~0.3g/日
【储藏】在避光、密封、阴凉处保存
【药物相互作用】与维生素C同服,可增加本品吸收;该药片在水和乙醇中溶解度不大。
现某学校化学实验小组,为了检测“速力菲”药片中亚铁元素的存在,设计了如下几组实验:
Ⅰ、理论设计方案:
 甲组同学按照设计的方案完成实验,但遗憾的是他们没有得到预期的实验结果,
甲组同学按照设计的方案完成实验,但遗憾的是他们没有得到预期的实验结果,
(1)甲组实验失败的可能原因:________________________________________________ 。
乙组同学认真思考了甲组实验失败的原因,模拟药片服用后在人体中溶解的变化过程,重新设计并完成下列实验:

(2)若试剂1为盐酸;则试剂2为________________________________。
丙组同学对乙组实验中最后红色褪去的现象产生了研究兴趣,探讨褪色的原因,根据微粒的组成,他们认为有两种可能的原因:
[Fe(SCN)]2+络离子中的三价铁被还原为亚铁
② 。
(3)请你对其中一种可能进行实验验证:________________________________
实验方案(用文字述):________________________________________________ ,
根据预期现象判断结论:________________________________________________________ 。
Ⅱ、(4)称量“速力菲”1.0 g,将其全部溶于稀硫酸中,配制成100.00 mL溶液,取出20.00 mL,用0.01000 mol/L的KMnO4溶液滴定。三次操作读数如下:
|
序号 |
V(KMnO4)初 |
V(KMnO4)终 |
V(KMnO4) |
|
1 |
2.24mL |
14.25mL |
12.01mL |
|
2 |
0.30mL |
12.72mL |
12.42mL |
|
3 |
0.50mL |
12.53 |
12.03mL |
计算:该补血药中含Fe2+的质量分数________________(保留小数点后二位小数)。