��Ŀ����
��12�֣�����ҩ������ơ�˵���鲿������ժ¼��
�����ÿƬ������������0.1g
���������״���ں�Fe2+34.0%��36.0%����ˮ��ʽ�Σ�Ϊ��Ĥ����Ƭ
����Ӧ֢������ȱ����ƶѪ֢Ԥ��������
�������÷�������Ԥ����0.1g���գ�������0.2g��0.4g���գ�С��Ԥ����30��60mg���գ�������0.1g��0.3g����
�����ء��ڱܹ⡢�ܷ⡢����������
��ҩ������á���ά����Cͬ���������ӱ�Ʒ���գ���ҩƬ��ˮ���Ҵ����ܽ�Ȳ���
��ijѧУ��ѧʵ��С�飬Ϊ�˼�⡰�����ơ�ҩƬ������Ԫ�صĴ��ڣ���������¼���ʵ�飺
��������Ʒ����� ����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
��1������ʵ��ʧ�ܵĿ���ԭ��________________________________________________��
����ͬѧ����˼���˼���ʵ��ʧ�ܵ�ԭ��ģ��ҩƬ���ú����������ܽ�ı仯���̣�������Ʋ��������ʵ�飺
��2�����Լ�1Ϊ������Լ�2Ϊ________________________________��
����ͬѧ������ʵ��������ɫ��ȥ������������о���Ȥ��̽����ɫ��ԭ����������ɣ�������Ϊ�����ֿ��ܵ�ԭ��
[Fe��SCN��]2+�������е�����������ԭΪ����
�� ��
��3�����������һ�ֿ��ܽ���ʵ����֤��________________________________
ʵ�鷽����������������________________________________________________,
����Ԥ�������жϽ��ۣ�________________________________________________________��
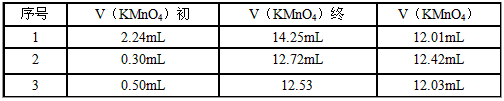
��4�������������ơ�1.0 g������ȫ������ϡ�����У����Ƴ�100.00 mL��Һ��ȡ��20.00 mL����0.01000 mol/L��KMnO4��Һ�ζ������β����������£�
| ��� | V��KMnO4���� | V��KMnO4���� | V��KMnO4�� |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |
���㣺�ò�Ѫҩ�к�Fe2+����������________________������С������λС������
��12�֣���1����ҩƬ��ˮ���ܽ�Ȳ��������ܽ�����Һ�м���������Fe2+���ӣ����Եò���Ԥ�ڵ�ʵ������2�֣�
��2�� KSCN��Һ��2�֣�
[Fe��SCN��]2+�������е�SCN����˫��ˮ������2�֣�
��3������ɫ����Һ����FeCl3��Һ��Һ��2�֣�
�����ɫ ���ۣ���ɫԭ������Ϊ�٣������ɫ ���ۣ���ɫԭ������Ϊ��
������ɫ����Һ�������KSCN��Һ ��2�֣�
�����ɫ ���ۣ���ɫԭ������Ϊ�ڣ������ɫ ���ۣ���ɫԭ������Ϊ��
��4��16.83%��2�֣�
����:��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���12�֣�����ҩ������ơ�˵���鲿������ժ¼��
�����ÿƬ������������0.1g
���������״���ں�Fe2+34.0%��36.0%����ˮ��ʽ�Σ�Ϊ��Ĥ����Ƭ
����Ӧ֢������ȱ����ƶѪ֢Ԥ��������
�������÷�������Ԥ����0.1g���գ�������0.2g��0.4g���գ�С��Ԥ����30��60mg���գ�������0.1g��0.3g����
�����ء��ڱܹ⡢�ܷ⡢����������
��ҩ������á���ά����Cͬ���������ӱ�Ʒ���գ���ҩƬ��ˮ���Ҵ����ܽ�Ȳ���
��ijѧУ��ѧʵ��С�飬Ϊ�˼�⡰�����ơ�ҩƬ������Ԫ�صĴ��ڣ���������¼���ʵ�飺
��������Ʒ�����  ����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
��1������ʵ��ʧ�ܵĿ���ԭ��________________________________________________ ��
����ͬѧ����˼���˼���ʵ��ʧ�ܵ�ԭ��ģ��ҩƬ���ú����������ܽ�ı仯���̣�������Ʋ��������ʵ�飺
��2�����Լ�1Ϊ������Լ�2Ϊ________________________________��
����ͬѧ������ʵ��������ɫ��ȥ������������о���Ȥ��̽����ɫ��ԭ����������ɣ�������Ϊ�����ֿ��ܵ�ԭ��
[Fe��SCN��]2+�������е�����������ԭΪ����
�� ��
��3�����������һ�ֿ��� ����ʵ����֤��________________________________
����ʵ����֤��________________________________
ʵ�鷽����������������________________________________________________ ,
����Ԥ�������жϽ��ۣ�________________________________________________________ ��
��4�������������ơ�1.0 g������ȫ������ϡ�����У����Ƴ�100.00 mL��Һ��ȡ��20.00 mL����0.01000 mol/L��KMnO4��Һ�ζ������β����������£�
| ��� | V��KMnO4���� | V��KMnO4���� | V��KMnO4�� |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |
��12�֣�����ҩ������ơ�˵���鲿������ժ¼��
�����ÿƬ������������0.1g
���������״���ں�Fe2+34.0%��36.0%����ˮ��ʽ�Σ�Ϊ��Ĥ����Ƭ
����Ӧ֢������ȱ����ƶѪ֢Ԥ��������
�������÷�������Ԥ����0.1g���գ�������0.2g��0.4g���գ�С��Ԥ����30��60mg���գ�������0.1g��0.3g����
�����ء��ڱܹ⡢�ܷ⡢����������
��ҩ������á���ά����Cͬ���������ӱ�Ʒ���գ���ҩƬ��ˮ���Ҵ����ܽ�Ȳ���
��ijѧУ��ѧʵ��С�飬Ϊ�˼�⡰�����ơ�ҩƬ������Ԫ�صĴ��ڣ���������¼���ʵ�飺
��������Ʒ�����
 ����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
��1������ʵ��ʧ�ܵĿ���ԭ��________________________________________________ ��
����ͬѧ����˼���˼���ʵ��ʧ�ܵ�ԭ��ģ��ҩƬ���ú����������ܽ�ı仯���̣�������Ʋ��������ʵ�飺

��2�����Լ�1Ϊ������Լ�2Ϊ________________________________��
����ͬѧ������ʵ��������ɫ��ȥ������������о���Ȥ��̽����ɫ��ԭ����������ɣ�������Ϊ�����ֿ��ܵ�ԭ��
[Fe��SCN��]2+�������е�����������ԭΪ����
�� ��
��3�����������һ�ֿ��ܽ���ʵ����֤��________________________________
ʵ�鷽����������������________________________________________________ ,
����Ԥ�������жϽ��ۣ�________________________________________________________ ��
��4�������������ơ�1.0 g������ȫ������ϡ�����У����Ƴ�100.00 mL��Һ��ȡ��20.00 mL����0.01000 mol/L��KMnO4��Һ�ζ������β����������£�
|
��� |
V��KMnO4���� |
V��KMnO4���� |
V��KMnO4�� |
|
1 |
2.24mL |
14.25mL |
12.01mL |
|
2 |
0.30mL |
12.72mL |
12.42mL |
|
3 |
0.50mL |
12.53 |
12.03mL |
���㣺�ò�Ѫҩ�к�Fe2+����������________________������С������λС������