��Ŀ����
����������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±��dz����¼���������ʵĵ���ƽ�ⳣ����
��ش��������⣺
(1)�����������У������������ǣߣߣߣߣߣߣ� (�û�ѧʽ��ʾ)��������ʹ������Һ��CH3COOH�ĵ���̶��������볣������IJ����ǣߣߣߣߣ� (�����)��
A�������¶�
B����ˮϡ��
C����������CH3COONa����
D��������������
(2)CH3COONH4��ˮ��Һ��________ (ѡ����ԡ������ԡ����ԡ�)��������__________________________________����Һ�и�����Ũ�ȴ�С�Ĺ�ϵ��_____________________________��
(3)���ʵ���1��1��NaCN��HCN�Ļ����Һ����pH��7������Һ�����ӵ�Ũ�ȴӴ�С����Ϊ_____________________________________��
(4)��֪һЩ��������ܶȻ��������±���
ij��ҵ��ˮ�к���Cu2����Pb2����Hg2������������˹�ҵ��ˮ�м�������� ��ȥ���ǡ�(ѡ�����)
��NaOH����FeS����Na2S
| ���� | ���볣��(Ka��Kb) |
| CH3COOH | 1��8��10��5 |
| HNO2 | 4��6��10��4 |
| HCN | 5��10��10 |
| HClO | 3��10��8 |
| NH3��H2O | 1��8��10��5 |
(1)�����������У������������ǣߣߣߣߣߣߣ� (�û�ѧʽ��ʾ)��������ʹ������Һ��CH3COOH�ĵ���̶��������볣������IJ����ǣߣߣߣߣ� (�����)��
A�������¶�
B����ˮϡ��
C����������CH3COONa����
D��������������
(2)CH3COONH4��ˮ��Һ��________ (ѡ����ԡ������ԡ����ԡ�)��������__________________________________����Һ�и�����Ũ�ȴ�С�Ĺ�ϵ��_____________________________��
(3)���ʵ���1��1��NaCN��HCN�Ļ����Һ����pH��7������Һ�����ӵ�Ũ�ȴӴ�С����Ϊ_____________________________________��
(4)��֪һЩ��������ܶȻ��������±���
| ���� | FeS | MnS | Cus | PbS | HgS | ZnS |
| Ksp | 6.3��10-18 | 2.5��10-13 | 1.3��10-36 | 3.4��10-28 | 6.4��10-55 | 1.6��10-24 |
��NaOH����FeS����Na2S
��1��HCN��B
��2�����ԡ�CH3COONH4����ˮ���ݵ���ƽ�ⳣ����CH3COO�����H����NH4+���OH������������ʵij̶�һ��������ˮ��Һ�е�H����OH��Ũ����ȣ������ԡ�c (NH4+)��c (CH3COO��)�� c (OH��)��c (H��)
��3�� c (Na��)�� c (CN��)�� c (OH��)�� c (H��)
(4)��
��2�����ԡ�CH3COONH4����ˮ���ݵ���ƽ�ⳣ����CH3COO�����H����NH4+���OH������������ʵij̶�һ��������ˮ��Һ�е�H����OH��Ũ����ȣ������ԡ�c (NH4+)��c (CH3COO��)�� c (OH��)��c (H��)
��3�� c (Na��)�� c (CN��)�� c (OH��)�� c (H��)
(4)��
(1)�ɱ������ݿ�֪HCN���Ka��С��������������
����ĵ��볣��ֻ���¶��йأ�Ҫ���䳣�����䣬���¶Ȳ��䣬�ų�A��
�ٽ�������룺CH3COOH CH3COO����H�����ɲ�ȡϡ�͵ķ�����B��ȷ
CH3COO����H�����ɲ�ȡϡ�͵ķ�����B��ȷ
��������CH3COONa���壬CH3COO��Ũ������ƽ�����ƣ����Ƶ���ƽ�⣬C����ȷ
�����������ᣬ��Ȼƽ�����ƣ�����ת���ʼ�С���ų�D
��2��CH3COONH4����ˮ���ݵ���ƽ�ⳣ����CH3COO�����H����NH4+���OH������������ʵij̶�һ��������ˮ��Һ�е�H����OH��Ũ����ȣ���Һ�����ԣ���c (OH��)��c (H��)���ٸ��ݵ���غ�c (NH4+)+ c (H��)��c (CH3COO��)+ c (OH��)����֪������Һ������Ũ�ȹ�ϵΪ��c (NH4+)��c (CH3COO��)�� c (OH��)��c (H��)
��3��NaCN��HCN�Ļ����Һ�У�������������ƽ�⣺
��CN����H2O HCN��OH�� ��HCN
HCN��OH�� ��HCN H����CN�� ��H2O
H����CN�� ��H2O H����OH��
H����OH��
��Һ�ʼ��ԣ���֪CN��ˮ��ʹ��Һ�ʼ��Եij������HCN����ʹ��Һ�����Եij̶ȣ�����Һ������Ũ��˳��Ϊc (Na��)�� c (CN��)�� c (OH��)�� c (H��)
(4)NaOH����ǿ��ʴ�ԣ�Na2S���������������ӣ����䱾��ˮ��Ҳ��ǿ���ԣ������ѡ��FeS��������Ũ�Ȼ�����Զ����CuS��HgS��PbS��������FeS��ʹ�ܽ�ƽ�������ƶ�������������ת������ʹ����Ҳ������������
����ĵ��볣��ֻ���¶��йأ�Ҫ���䳣�����䣬���¶Ȳ��䣬�ų�A��
�ٽ�������룺CH3COOH
 CH3COO����H�����ɲ�ȡϡ�͵ķ�����B��ȷ
CH3COO����H�����ɲ�ȡϡ�͵ķ�����B��ȷ��������CH3COONa���壬CH3COO��Ũ������ƽ�����ƣ����Ƶ���ƽ�⣬C����ȷ
�����������ᣬ��Ȼƽ�����ƣ�����ת���ʼ�С���ų�D
��2��CH3COONH4����ˮ���ݵ���ƽ�ⳣ����CH3COO�����H����NH4+���OH������������ʵij̶�һ��������ˮ��Һ�е�H����OH��Ũ����ȣ���Һ�����ԣ���c (OH��)��c (H��)���ٸ��ݵ���غ�c (NH4+)+ c (H��)��c (CH3COO��)+ c (OH��)����֪������Һ������Ũ�ȹ�ϵΪ��c (NH4+)��c (CH3COO��)�� c (OH��)��c (H��)
��3��NaCN��HCN�Ļ����Һ�У�������������ƽ�⣺
��CN����H2O
 HCN��OH�� ��HCN
HCN��OH�� ��HCN H����CN�� ��H2O
H����CN�� ��H2O H����OH��
H����OH����Һ�ʼ��ԣ���֪CN��ˮ��ʹ��Һ�ʼ��Եij������HCN����ʹ��Һ�����Եij̶ȣ�����Һ������Ũ��˳��Ϊc (Na��)�� c (CN��)�� c (OH��)�� c (H��)
(4)NaOH����ǿ��ʴ�ԣ�Na2S���������������ӣ����䱾��ˮ��Ҳ��ǿ���ԣ������ѡ��FeS��������Ũ�Ȼ�����Զ����CuS��HgS��PbS��������FeS��ʹ�ܽ�ƽ�������ƶ�������������ת������ʹ����Ҳ������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
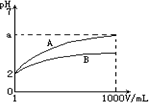
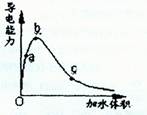
 �ʵ���Ũ����ͬʱ
�ʵ���Ũ����ͬʱ ��������Һ��ˮ�ĵ���̶��ɴ�С��˳����____________������a��b��c��ʾ����ͬ��
��������Һ��ˮ�ĵ���̶��ɴ�С��˳����____________������a��b��c��ʾ����ͬ�� aOH�������ɴ�С��˳����___________��
aOH�������ɴ�С��˳����___________�� ��c(H��)�ɴ�С��˳����_________________��
��c(H��)�ɴ�С��˳����_________________�� CH3COO�D+H��
CH3COO�D+H��