��Ŀ����
��13�֣� CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�������CuSO4��5H2O��ʵ�����Ʊ�����ͼ��
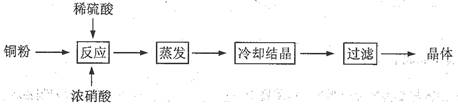
�����������������գ�
��1����ͭ�۵�ϡ�����еμ�Ũ���ᣬ��ͭ���ܽ�ʱ���Թ۲쵽��ʵ������ �� ��
��2����ͬѧ��Ϊ���ַ����������ж��ĵ���������, �����þ�����ܻ�������ͭ�����齫Ũ���ỻ��������������������أ�����ͬ�У���ѡ����ʵ��Լ�������з�Ӧ�����з�Ӧ������Ӧע������
__Cu + __H2SO4 + __________�� __CuSO4 +_______________
��3����֪��CuSO4+2NaOH=Cu(OH)2��+ Na2SO4
��Է���������CuSO4��160 H2O��18
��ȡ0.1000 g�ᴿ���CuSO4��5H2O��������ƿ�У�����0.1000 mol/L����������Һ28.00 mL����Ӧ��ȫ����������������0.1000 mol/L����ζ����յ㣬��������20.16 mL����������к�CuSO4��5H2O����������Ϊ ��
��4�������ζ��У��ζ�����ע������֮ǰ����������ˮϴ�������� ��
��5����������������ⶨCuSO4��5H2O�ĺ�����������в��裺
�ٳ�ȡ��Ʒa g�ڼ�ˮ�ܽ�ۼ��Ȼ�����Һ�������ܹ��ˡ� ������ݳ������ù���b g��
�ڹ���ǰ����Ҫ�����Ƿ������ȫ���������
��a g�������к�CuSO4��5H2O������Ϊ g ���ú�b�Ĵ���ʽ��ʾ����
[���ԭ��������H-1 O-16 S-32 Ba-137]

�����������������գ�
��1����ͭ�۵�ϡ�����еμ�Ũ���ᣬ��ͭ���ܽ�ʱ���Թ۲쵽��ʵ������ �� ��
��2����ͬѧ��Ϊ���ַ����������ж��ĵ���������, �����þ�����ܻ�������ͭ�����齫Ũ���ỻ��������������������أ�����ͬ�У���ѡ����ʵ��Լ�������з�Ӧ�����з�Ӧ������Ӧע������
__Cu + __H2SO4 + __________�� __CuSO4 +_______________
��3����֪��CuSO4+2NaOH=Cu(OH)2��+ Na2SO4
��Է���������CuSO4��160 H2O��18
��ȡ0.1000 g�ᴿ���CuSO4��5H2O��������ƿ�У�����0.1000 mol/L����������Һ28.00 mL����Ӧ��ȫ����������������0.1000 mol/L����ζ����յ㣬��������20.16 mL����������к�CuSO4��5H2O����������Ϊ ��
��4�������ζ��У��ζ�����ע������֮ǰ����������ˮϴ�������� ��
��5����������������ⶨCuSO4��5H2O�ĺ�����������в��裺
�ٳ�ȡ��Ʒa g�ڼ�ˮ�ܽ�ۼ��Ȼ�����Һ�������ܹ��ˡ� ������ݳ������ù���b g��
�ڹ���ǰ����Ҫ�����Ƿ������ȫ���������
��a g�������к�CuSO4��5H2O������Ϊ g ���ú�b�Ĵ���ʽ��ʾ����
[���ԭ��������H-1 O-16 S-32 Ba-137]
��1����Һ����ɫ���к���ɫ��������2��
 ��2��Cu + H2SO4 + H2O2= CuSO4 +2H2O��
��2��Cu + H2SO4 + H2O2= CuSO4 +2H2O��
2Cu + 2H2SO4 + O2==2CuSO4 ++2H2O 2��
��3��98% 2��
��4����������Һ��ϴ2��3�� 2��
��5��ϴ��1�� ���ϲ���Һ�м����μӼ��Ȼ�����Һ���۲�����������2��
250b/233 g 2��
 ��2��Cu + H2SO4 + H2O2= CuSO4 +2H2O��
��2��Cu + H2SO4 + H2O2= CuSO4 +2H2O��2Cu + 2H2SO4 + O2==2CuSO4 ++2H2O 2��
��3��98% 2��
��4����������Һ��ϴ2��3�� 2��
��5��ϴ��1�� ���ϲ���Һ�м����μӼ��Ȼ�����Һ���۲�����������2��
250b/233 g 2��
��

��ϰ��ϵ�д�
�����Ŀ
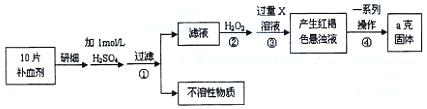
 ��ش��������⣺
��ش��������⣺ ��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��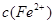 �ı仯��ͼ��ʾ���������ӷ���ʽ�������( )
�ı仯��ͼ��ʾ���������ӷ���ʽ�������( )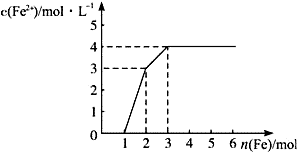

 05 mol/L��NaOH��Һ������һ����Һ�еμ�0.600 mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������
05 mol/L��NaOH��Һ������һ����Һ�еμ�0.600 mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ����������������� Һ������仯����ͼ��ʾ��
Һ������仯����ͼ��ʾ��
 ����ַ�Ӧ���ڱ�״��������NO�������ʣ��������������±���������Ļ�ԭ����ֻ��NO����
����ַ�Ӧ���ڱ�״��������NO�������ʣ��������������±���������Ļ�ԭ����ֻ��NO����