��Ŀ����
ͭ��ͭ�Ļ������ж���Ӧ�ã���ش�����������⣺
��1���������ַ����������Ʊ�CuSO4
��2Cu+O2 2CuO���������� CuO+H2SO4=CuSO4+H2O������ ��Cu+2H2SO4��Ũ��
2CuO���������� CuO+H2SO4=CuSO4+H2O������ ��Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
����ij����������CuSO4����ѡ��һ�ַ�����˵������______��
��2����֪ͭ��ϡ�����Ӧ������ͭƬ��ϡ�����г�ʱ�����ʱ��Һ�����ɫ�����û�ѧ����ʽ��ʾԭ��______��
���ͭƬ��ϡ�����м���ʱ���뼸��H2O2����ҺҲ��ܿ����ɫ���������ӷ���ʽ��ʾԭ��______��
��3����֪��2Cu��s��+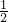 O2��g��=Cu2O��s������H1=-12.0kJ/mol
O2��g��=Cu2O��s������H1=-12.0kJ/mol
2Cu��s��+S��s��=Cu2S��s������H2=-79.5kJ/mol
S��s��+O2 ��g��=SO2��g������H3=-296.83kJ/mol
�� Cu2S��s��+2Cu2O��s��=6Cu��s��+SO2��g������H4=______kJ/mol
��4��ij��ɫ����ݷ�����Cu2S��CuS��ɣ�
��֪ Cu2S���Ժ����ᷴӦ������ƽ���л�ѧ��Ӧ����ʽ����Cu2S+��HNO3 ��CuSO4+��Cu��NO3��2+��NO��+��______
��CuSO4+��Cu��NO3��2+��NO��+��______
��ѧ��ȤС��ͬѧ��������ɫ����ijɷ֣����������ʵ�鷽�����ȳ�ȡ��Ʒm1g��Ȼ���ּ��ȷ�Ӧ��������CuO����Ϊm2g������Ʒ�к�Cu2S______g��
�⣺��1����������Cu��H2SO4��������100%��������Ⱦ����������Cu��������100%��H2SO4��������50%���Ҳ�����Ⱦ��ʴ𰸣�ѡ����ԭ�������ʸߣ���Ⱦ�٣�
��2������Һ������������������ͭ��Ӧ��������ͭ��2Cu+O2 2CuO������ͭ�������ᷴӦ��CuO+H2SO4=CuSO4+H2O����ʽ��ӣ�2Cu+O2+2H2SO4
2CuO������ͭ�������ᷴӦ��CuO+H2SO4=CuSO4+H2O����ʽ��ӣ�2Cu+O2+2H2SO4 2CuSO4+2H2O�������ʱ���뼸��
2CuSO4+2H2O�������ʱ���뼸��
H2O2����ҺҲ��ܿ����ɫ��˵��H2O2�ܽ�ͭ������ͭ���ӣ����ӷ�ӦΪCu+H2O2+2H+ Cu2++2H2O���ʴ𰸣�2Cu+O2+2H2SO4
Cu2++2H2O���ʴ𰸣�2Cu+O2+2H2SO4 2CuSO4+2H2O��Cu+H2O2+2H+
2CuSO4+2H2O��Cu+H2O2+2H+ Cu2++2H2O��
Cu2++2H2O��
��3����֪��2Cu��s��+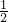 O2��g��=Cu2O��s������H1=-12.0kJ/mol ��
O2��g��=Cu2O��s������H1=-12.0kJ/mol ��
2Cu��s��+S��s��=Cu2S��s������H2=-79.5kJ/mol ��
S��s��+O2 ��g��=SO2��g������H3=-296.83kJ/mol ��
���ݸ�˹���ɢ�-�١�2-�ڿɵ�Cu2S��s��+2Cu2O��s��=6Cu��s��+SO2��g������H4=-193.33kJ/mol��
�ʴ�Ϊ��-193.33��
��4�����ݻ��ϼ�����������ȿ�֪��
Cu2S Cu��+1��+2��2 ����10��3
S��-2��+6��8
HNO3 N��+5��+2��3��10
����ƽ��Ļ�ѧ��ӦΪ3Cu2S+16HNO3 3CuSO4+3Cu��NO3��2+10NO��+8H2O��
3CuSO4+3Cu��NO3��2+10NO��+8H2O��
�ʴ𰸣�3��16��3��3��10��8H2O��
��5����Cu2S ������Ϊx��CuS ������Ϊy��
Cu2S��2CuO CuS��CuO
160 160 96 80
x x y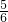 y
y
���������飺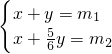
��ã�x=6m2-5m1��y=6m1-6m2��
�ʴ�Ϊ��6m2-5m1��
��������1������ԭ�������ʺͻ����ĽǶȣ�
��2��������Һ���������μӷ�Ӧ��H2O2����������������
��3�����ݸ�˹���ɼ��㷴Ӧ�ȣ�
��4������������ԭ��Ӧ�л��ϼ�����������ȼ������غ�����ƽ��
��5������Cu2S��CuS���Ⱥ����ն����CuO��������������⣮
������������Ҫ������ͭ�Ļ�ѧ���ʣ�ͬʱҲ�����˸�˹���ɵ����ã�������ԭ��Ӧ��������������ۺ��ԣ�
��2������Һ������������������ͭ��Ӧ��������ͭ��2Cu+O2
 2CuO������ͭ�������ᷴӦ��CuO+H2SO4=CuSO4+H2O����ʽ��ӣ�2Cu+O2+2H2SO4
2CuO������ͭ�������ᷴӦ��CuO+H2SO4=CuSO4+H2O����ʽ��ӣ�2Cu+O2+2H2SO4 2CuSO4+2H2O�������ʱ���뼸��
2CuSO4+2H2O�������ʱ���뼸��H2O2����ҺҲ��ܿ����ɫ��˵��H2O2�ܽ�ͭ������ͭ���ӣ����ӷ�ӦΪCu+H2O2+2H+
 Cu2++2H2O���ʴ𰸣�2Cu+O2+2H2SO4
Cu2++2H2O���ʴ𰸣�2Cu+O2+2H2SO4 2CuSO4+2H2O��Cu+H2O2+2H+
2CuSO4+2H2O��Cu+H2O2+2H+ Cu2++2H2O��
Cu2++2H2O�� ��3����֪��2Cu��s��+
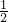 O2��g��=Cu2O��s������H1=-12.0kJ/mol ��
O2��g��=Cu2O��s������H1=-12.0kJ/mol ��2Cu��s��+S��s��=Cu2S��s������H2=-79.5kJ/mol ��
S��s��+O2 ��g��=SO2��g������H3=-296.83kJ/mol ��
���ݸ�˹���ɢ�-�١�2-�ڿɵ�Cu2S��s��+2Cu2O��s��=6Cu��s��+SO2��g������H4=-193.33kJ/mol��
�ʴ�Ϊ��-193.33��
��4�����ݻ��ϼ�����������ȿ�֪��
Cu2S Cu��+1��+2��2 ����10��3
S��-2��+6��8
HNO3 N��+5��+2��3��10
����ƽ��Ļ�ѧ��ӦΪ3Cu2S+16HNO3
 3CuSO4+3Cu��NO3��2+10NO��+8H2O��
3CuSO4+3Cu��NO3��2+10NO��+8H2O���ʴ𰸣�3��16��3��3��10��8H2O��
��5����Cu2S ������Ϊx��CuS ������Ϊy��
Cu2S��2CuO CuS��CuO
160 160 96 80
x x y
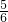 y
y ���������飺
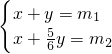
��ã�x=6m2-5m1��y=6m1-6m2��
�ʴ�Ϊ��6m2-5m1��
��������1������ԭ�������ʺͻ����ĽǶȣ�
��2��������Һ���������μӷ�Ӧ��H2O2����������������
��3�����ݸ�˹���ɼ��㷴Ӧ�ȣ�
��4������������ԭ��Ӧ�л��ϼ�����������ȼ������غ�����ƽ��
��5������Cu2S��CuS���Ⱥ����ն����CuO��������������⣮
������������Ҫ������ͭ�Ļ�ѧ���ʣ�ͬʱҲ�����˸�˹���ɵ����ã�������ԭ��Ӧ��������������ۺ��ԣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
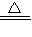 2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ��
2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ��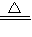 CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O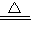 2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ��
2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ��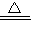 CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O