��Ŀ����
19��ʵ��������ϩ��������Ϊ�¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������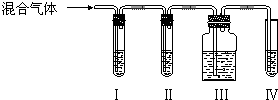
��1��д��ʵ������ȡ��ϩ�Ļ�ѧ����ʽCH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O
��2����װ���п�ʢ�ŵ��Լ��ǣ����������й��Լ����������ո��ڣ�
��A����B����A������D��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D����ˮ
��3����˵������������ڵ������Ǣ���Ʒ����Һ��ɫ��
��4��ʹ��װ�ã���Ŀ���dz�ȥ�����������������ϩ�ļ��飮
��5��ʹ��װ�ã���Ŀ������֤SO2�Ƿ������
��6��ȷ֤����ϩ���������Ʒ�첻��ɫ����IV����Һ��ɫ��dz����ɫ��
��7����������ʵ��ʱ����Ҫʹ���¶ȼƣ��ش��������⣺
��ȡ��ϩʱ���¶ȼ�ˮ����λӦ���ڷ�Ӧ�����Һ�����£�
ʯ�ͷ���ʱ���¶ȼ�ˮ����λӦ����������ƿ��֧�ܴ���
���� �Ҵ���Ũ���ᷴӦ�Ʊ���ϩ���¶ȹ����������������������װ�ÿ�֪��I�м������������Ʒ����Һ��II��NaOH��ȥ����������Ʒ�������������������������ˮ����������������Һ����ɫ������ϩ���Դ������
��� �⣺��1��ʵ������ȡ��ϩ�Ļ�ѧ����ʽΪCH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O���ʴ�Ϊ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
��2����ʵ��Ŀ�Ŀ�֪��ȷ�������������������ϩ�Ͷ�������I���Լ�ΪA��Ʒ����Һ����II���Լ�ΪB��NaOH��Һ���������Լ�ΪA��Ʒ����Һ���������Լ�ΪD����ˮ�����ʴ�Ϊ��A��B��A��D��
��3����˵����������ڵ�������װ��I��Ʒ����Һ��ɫ���ʴ�Ϊ��װ��I��Ʒ����Һ��ɫ��
��4��ʹ��װ�ã���Ŀ���dz�ȥ�����������������ϩ�ļ��飬�ʴ�Ϊ����ȥ�����������������ϩ�ļ��飻
��5��ʹ��װ�ã���Ŀ���Ǽ�����������Ƿ�������ʴ�Ϊ��������������Ƿ������
��6��ȷ֤����ϩ������Ϊװ�â���Ʒ�첻��ɫ��װ�â�����ˮ��ɫ���ʴ�Ϊ��װ�â���Ʒ�첻��ɫ��װ�â�����ˮ��ɫ��
��7����ȡ��ϩʱ���ⶨ��ӦҺ���¶ȣ����¶ȼ�ˮ����λӦ������Һ�У���ʯ�ͷ���ʱ���ⶨ��ֵ��¶ȣ����¶ȼ�ˮ����λӦ����������ƿ֧�ܿڴ���
�ʴ�Ϊ����Ӧ�����Һ�����£�������ƿ֧�ܿڴ���
���� ���⿼������ʵ�鷽������ƣ�Ϊ��Ƶ���㣬������ѧ���ķ�����ʵ�������Ŀ��飬�������ʵ����ʡ����鼰ʵ��װ�õ�����Ϊ���Ĺؼ���ע�������ϩӦ�ų���������ĸ��ţ���Ŀ�ѶȲ���

| A�� | 1��3-����ϩ��CH2=CH-CH=CH2��������ʵ�����Br2�����ӳɷ�Ӧ | |
| B�� | 2-�ȶ��飨 ����NaOH�Ҵ���Һ���ȷ�����ȥHCl���ӵķ�Ӧ ����NaOH�Ҵ���Һ���ȷ�����ȥHCl���ӵķ�Ӧ | |
| C�� | �ױ���һ�����������������ᷢ��������Ӧ | |
| D�� | �Ҵ���HBr������ȡ����Ӧ |
| A�� | 22.4 L�����У�һ������2mol��ԭ�� | |
| B�� | 80 g NaOH�ܽ���1 Lˮ�У��õ���Һ�����ʵ���Ũ��Ϊ2mol/L | |
| C�� | 18 gˮ�ڱ�״���µ����ԼΪ22.4L | |
| D�� | ��״���£�20 mLNH3��60 mLO2�������Ӹ�����Ϊ1��3 |

| A�� | �����ʷ���ʽΪC13H16O4 | |
| B�� | ����FeCl3��Һ������ɫ��Ӧ | |
| C�� | ��һ�������¿ɷ����ӳɡ�ȡ������ȥ��Ӧ | |
| D�� | 1 mol�������������2mol NaOH��Ӧ |
| A�� | 2HgO $\frac{\underline{\;\;��\;\;}}{\;}$2Hg+O2�� | B�� | 2A12O3 $\frac{\underline{\;���\;}}{\;}$4Al+3O2�� | ||
| C�� | H2+Na2O $\frac{\underline{\;����\;}}{\;}$2Na+H2O | D�� | 4 CO+Fe3O4$\frac{\underline{\;����\;}}{\;}$3Fe+4CO2 |
| A�� | �е㣺NH3��PH3��AsH3 | B�� | �۵㣺MgO��NaCl��KCl | ||
| C�� | ���ԣ�HClO4��H2SO4��H3PO4 | D�� | ���ԣ�NaOH��Mg��OH��2��Al��OH��3 |
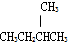 D��
D�� ��
�� E��CH3CH2CH2CH3��
E��CH3CH2CH2CH3��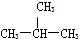




 ��1�������Ͻ����κ��Է���������ԭ��Ӧ��������Ƴ�ԭ��أ������÷�Ӧ��Cu+2Ag+=2Ag+Cu2+������һ����ѧ��أ�����������̼�������ش��������⣺
��1�������Ͻ����κ��Է���������ԭ��Ӧ��������Ƴ�ԭ��أ������÷�Ӧ��Cu+2Ag+=2Ag+Cu2+������һ����ѧ��أ�����������̼�������ش��������⣺