��Ŀ����
(һ) ��1������Ҳ��һ�����ȼ�ϣ�������ȫȼ��ʱ��Ч�ʽ��Ͳ�������ж����������Ⱦ��
��֪�� CH4(g) + 2O2(g) �� CO2(g) + 2H2O(l) ��H1���D890.3 kJ/mol
2CO (g) + O2(g) �� 2CO2(g) ��H2���D566.0 kJ/mol
����鲻��ȫȼ������һ����̼��Һ̬ˮʱ����Ч��ֻ����ȫȼ��ʱ��________��������������1λС������
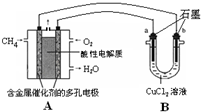
��2������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��50 mL 2 mol/L���Ȼ�ͭ��Һ��װ��ʾ��ͼ��
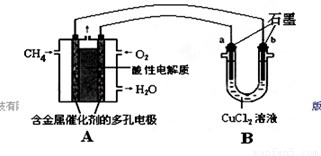
��ش�
�ټ���ȼ�ϵ�صĸ�����Ӧʽ��________��
�ڵ���·����0.1 mol����ͨ��ʱ��________���a����b����������________g��
�������±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ�
�ܶȻ�Ksp (25��)��
|
����� |
ƽ�ⷽ��ʽ |
ƽ�ⳣ��K |
Ksp |
|
CH3COOH |
CH3COOH |
1.76��10-5 |
|
|
H2CO3 |
H2CO3 HCO3- |
K1��4.31��10-7 K2��5.61��10-11 |
|
|
C6H5OH |
C6H5OH |
1.1��10-10 |
|
|
H3PO4 |
H3PO4 H2PO4- HPO42- |
K1��7.52��10-3 K2��6.23��10-8 K3��2.20��10-13 |
|
|
NH3��H2O |
NH3��H2O |
1.76��10-5 |
|
|
BaSO4 |
BaSO4 |
|
1.07��10-10 |
|
BaCO3 |
BaCO3 |
|
2.58��10-9 |
�ش��������⣺
��1�����ϱ�����������CH3COOH ��HCO3- ��C6H5OH ��H2PO4- ���ɿ����ᣬ������������ǿ������˳��Ϊ__________________________(����)��
(2)25��ʱ�����������Ũ�ȵĴ���Ͱ�ˮ��ϣ����Һ�У�c(CH3COO-)______c(NH4+)��(�����������������)
��3��25��ʱ����10ml 0.01mol/L������Һ�еμ�Vml 0.01mol/L��ˮ�������Һ������Ũ�ȹ�ϵ��ȷ����( )��
A�������ҺpH��7����V��10
B�������ҺpH��7����c((NH4+) ��c (C6H5O-) ��c (H+)��c (OH��)
C��V=10ʱ�����Һ��ˮ�ĵ���̶�С��10ml 0.01mol/L������Һ��ˮ�ĵ���̶�
D��V=5ʱ��2c(NH3��H2O)+ 2 c (NH4+)= c (C6H5O-)+ c (C6H5OH)
��4������ͼ��ʾ����T1��T2�����¶�������BaSO4��ˮ�еij����ܽ�ƽ�����ߣ��ش��������⣺
����T1�¶�ʱBaSO4�ij����ܽ�ƽ�����ߣ�����˵������ȷ����( )
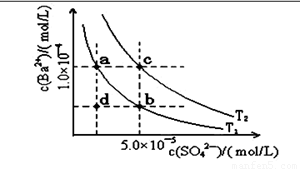
A������Na2SO4��ʹ��Һ��a���Ϊb��
B����T1�����Ϸ�����(��������)����һ��ʱ�� ����BaSO4��������
C�������ܼ�����ʹ��Һ��d���Ϊ������a�� b֮���ijһ��(����a��b)
D�����¿�ʹ��Һ��b���Ϊd��
�� ��1��0.7 ��2�֣� ��2�� ��CH4-8e-+2H2O=CO2+8H+ �� b 3.2 ����2�֣�
�棨1���٢ܢۢ� ��2�֣� ��2�� = ��1�֣� ��3�� D ��2�֣� ��4�� D ��2�֣�
��������
����������壨1�����鲻��ȫȼ�յ��Ȼ�ѧ����ʽΪ: CH4(g) + O2(g) �� CO(g)
+ 2H2O(l) ��H���D607.3
kJ/mol, ����鲻��ȫȼ������һ����̼��Һ̬ˮʱ����Ч��ֻ����ȫȼ��ʱ��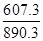 =0.7�� ��2���ٵ����Ϊ���Թʸ�����ӦʽΪ��CH4-8e-+2H2O=CO2+8H+
��Cu2+�������ŵ缴b����ÿͨ��0.1mol���ӣ���0.5molCu2+�ŵ磬��3.2g �棨1�� KֵԽ������Խǿ ��2��25��ʱ����Ͱ�ˮ�ĵ���̶���ͬ���ʽ��������Ũ�ȵĴ���Ͱ�ˮ��Ϻ���Һ�����ԣ��ʻ��Һ��c(CH3COO-)=c(NH4+)����3����ΪNH3��H2O�ĵ���̶�Զ����C6H5OH������10ml 0.01mol/L������Һ�еμ�Vml
0.01mol/L��ˮ����ˮ���������Ҫ��10mL��Һ��pH>7,A ����B����ѭ����غ㣬����V=10ʱ�����ҺΪ�������Һ�������ˮ��ٽ�ˮ�ĵ��룬�ʻ��Һ��ˮ�ĵ���̶ȴ���10ml 0.01mol/L������Һ��ˮ�ĵ���̶ȣ�C����4�������¶ȣ�BaSO4�ĵ���ƽ�������ƶ���c��SO42-����c(Ba2+)������D����
=0.7�� ��2���ٵ����Ϊ���Թʸ�����ӦʽΪ��CH4-8e-+2H2O=CO2+8H+
��Cu2+�������ŵ缴b����ÿͨ��0.1mol���ӣ���0.5molCu2+�ŵ磬��3.2g �棨1�� KֵԽ������Խǿ ��2��25��ʱ����Ͱ�ˮ�ĵ���̶���ͬ���ʽ��������Ũ�ȵĴ���Ͱ�ˮ��Ϻ���Һ�����ԣ��ʻ��Һ��c(CH3COO-)=c(NH4+)����3����ΪNH3��H2O�ĵ���̶�Զ����C6H5OH������10ml 0.01mol/L������Һ�еμ�Vml
0.01mol/L��ˮ����ˮ���������Ҫ��10mL��Һ��pH>7,A ����B����ѭ����غ㣬����V=10ʱ�����ҺΪ�������Һ�������ˮ��ٽ�ˮ�ĵ��룬�ʻ��Һ��ˮ�ĵ���̶ȴ���10ml 0.01mol/L������Һ��ˮ�ĵ���̶ȣ�C����4�������¶ȣ�BaSO4�ĵ���ƽ�������ƶ���c��SO42-����c(Ba2+)������D����
���㣺��ѧ��Ӧ�������仯��˵��Һ���ۺϿ���

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д� CH3COO-��H+
CH3COO-��H+ C6H5O-��H+
C6H5O-��H+ NH4+��OH-
NH4+��OH-
 ��2013?����һģ����������Դ��ʹ�����ȼ�ϣ����Դﵽ�����Ч��������Ⱦ��Ŀ�ģ�
��2013?����һģ����������Դ��ʹ�����ȼ�ϣ����Դﵽ�����Ч��������Ⱦ��Ŀ�ģ�