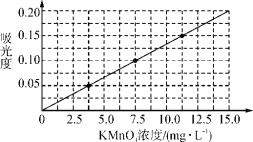
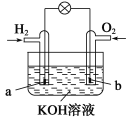
ƒøƒ⁄»ð
°æƒø°øA°¢B°¢C°¢DÀƒŒÔ÷ ”–»Áœ¬◊™ªØπÿœµ(∑¥”¶Ãıº˛∫Õ≤ø∑÷≤˙ŒÔ“—¬‘»•)£∫A![]() B
B![]() C
C![]() D£¨ªÿ¥œ¬¡–Œ £∫
D£¨ªÿ¥œ¬¡–Œ £∫
(1)»ÙAŒ™∆¯Ã¨«‚ªØŒÔ∫ÕDƒÐÕ®π˝ªØ∫œ∑¥”¶…˙≥…“ª÷÷—Œ£¨‘Ú
¢ŸºÚ ˆºÏ—È∏√—Œ÷–—Ù¿Î◊”µƒ∑Ω∑®£∫_____________________________________£ª
¢⁄–¥≥ˆ µ—È “÷∆»°AµƒªØ—ß∑Ω≥Ã Ω£∫______________________________________£ª
¢€–¥≥ˆ”…A…˙≥…BµƒªØ—ß∑Ω≥Ã Ω£∫______________________________________£ª
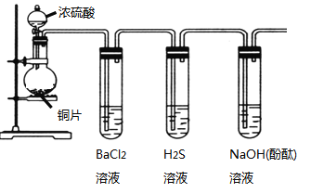
¢Ðƒ≥Õ¨—ß”√∏…‘Ôµƒ‘≤µ◊…’∆ø ’ºØ“ª∆øA∆¯Ã£¨”√µŒ»Î∑”ÙµƒÀÆ◊ˆ≈Á»™ µ—È£¨ƒÐπ€≤ϵΩ√¿¿ˆµƒ∫Ï…´≈Á»™°£”√∑Ω≥à ΩΩ‚ Õ≈Á»™≥ ∫Ï…´µƒ‘≠“Ú£∫____________________°£
(2)»ÙA”ÎB∑¥”¶ƒÐ…˙≥…“ª÷÷µ≠ª∆…´πÃõ•÷ £¨–¥≥ˆ∏√∑¥”¶µƒªØ—ß∑Ω≥Ã Ω£∫ ________________________________________________________________°£
(3)»ÙA «Ω Ùµ•÷ £¨C «“ª÷÷µ≠ª∆…´πÃ㨖¥≥ˆC…˙≥…Dµƒ¿Î◊”∑Ω≥Ã Ω£∫______________°£
°æ¥∞∏°ø»°…Ÿ¡ø¥˝≤‚“∫”⁄ ‘πÐ÷–º”»Î«‚—ıªØƒ∆»Ð“∫≤¢º”»»£¨»Á”– π ™»Ûµƒ∫Ï…´ Ø»Ô ‘÷Ω±‰¿∂µƒ∆¯ÃÂ∑≈≥ˆø…»∑∂®”–Ôß∏˘¿Î◊” Ca(OH)2+2NH4Cl![]() CaCl2£´2NH3°¸+2H2O 4NH3£´5O2
CaCl2£´2NH3°¸+2H2O 4NH3£´5O2![]() 4NO£´6H2O NH3£´H2O
4NO£´6H2O NH3£´H2O![]() NH3°§H2O
NH3°§H2O![]() NH4+£´OH- SO2+2H2S=3S°˝+2H2O 2Na2O2+2H2O=4Na+£´4OH-+O2°¸
NH4+£´OH- SO2+2H2S=3S°˝+2H2O 2Na2O2+2H2O=4Na+£´4OH-+O2°¸
°æΩ‚Œˆ°ø
(1)AŒ™∆¯Ã¨«‚ªØŒÔ∫ÕDƒÐÕ®π˝ªØ∫œ∑¥”¶…˙≥…“ª÷÷—Œ£¨‘ÚAŒ™NH3£¨BŒ™NO£¨CŒ™NO2£¨DŒ™NH4NO3£ª
(2)µ≠ª∆…´πÃõ•÷ Œ™S£¨‘ÚAŒ™H2S£¨BŒ™SO2£¨CŒ™SO3£¨DŒ™H2SO4£ª
(3)»ÙA «Ω Ùµ•÷ £¨C «“ª÷÷µ≠ª∆…´πÃ㨔¶Œ™Na2O2£¨‘ÚAŒ™Na£¨BŒ™Na2O£¨DŒ™NaOH°£
(1)AŒ™∆¯Ã¨«‚ªØŒÔ∫ÕDƒÐÕ®π˝ªØ∫œ∑¥”¶…˙≥…“ª÷÷—Œ£¨‘ÚAŒ™NH3£¨BŒ™NO£¨CŒ™NO2£¨DŒ™NH4NO3°£
¢Ÿ∏√—Œ÷–—Ù¿Î◊”Œ™NH4+£¨ºÏ—ÈNH4+∑Ω∑® «»°…Ÿ¡ø¥˝≤‚“∫”⁄ ‘πÐ÷–º”»Î«‚—ıªØƒ∆»Ð“∫≤¢º”»»£¨»Ù”– π ™»Ûµƒ∫Ï…´ Ø»Ô ‘÷Ω±‰¿∂µƒ∆¯Ã£¨Àµ√˜∑≈≥ˆµƒ∆¯ÃÂŒ™NH3£¨‘Ú‘≠πÃÃÂ÷–∫¨”–NH4+£ª
¢⁄‘⁄ µ—È “ø…“‘ø…”√«‚—ıªØ∏∆∫Õ¬»ªØÔßπÃêÏ∫œº”»»÷∆±∏∞±∆¯£¨∑¥”¶∑Ω≥Ã ΩŒ™Ca(OH)2+2NH4Cl![]() CaCl2+2NH3°¸+2H2O£ª
CaCl2+2NH3°¸+2H2O£ª
¢€∞±∆¯‘⁄¥þªØÃıº˛œ¬ø……˙≥…NO∫ÕÀÆ£¨∑Ω≥Ã ΩŒ™4NH3+5O2![]() 4NO+6H2O£ª
4NO+6H2O£ª
¢Ð∞±∆¯“◊»Ð”⁄ÀÆ£¨”ÎÀÆ∑¥”¶…˙≥…“ªÀÆ∫œ∞±£¨“ªÀÆ∫œ∞± «»ıµÁΩ‚÷ £¨µÁ¿Î≤˙…˙NH4+∫ÕOH-£¨ π»Ð“∫≥ ºÓ–‘£¨∑¥”¶‘≠¿ÌŒ™£∫NH3+H2O![]() NH3H2O
NH3H2O![]() NH4++OH-£ª
NH4++OH-£ª
(2)µ≠ª∆…´πÃõ•÷ Œ™S£¨‘ÚAŒ™H2S£¨BŒ™SO2£¨CŒ™SO3£¨DŒ™H2SO4£¨…˙≥…¡Úµƒ∑Ω≥Ã ΩŒ™SO2+2H2S=3S+2H2O£ª
(3) »ÙA «Ω Ùµ•÷ £¨C «“ª÷÷µ≠ª∆…´πÃ㨑ÚC”¶Œ™Na2O2£¨‘ÚAŒ™Na£¨BŒ™Na2O£¨DŒ™NaOH£¨π˝—ıªØƒ∆∫ÕÀÆ∑¥”¶…˙≥…«‚—ıªØƒ∆∫Õ—ı∆¯£¨∑¥”¶µƒ¿Î◊”∑Ω≥Ã ΩŒ™2Na2O2+2H2O=4Na++4OH-+O2°¸°£

 ø∆—ß µ—ȪÓ∂Ø≤·œµ¡–¥∞∏
ø∆—ß µ—ȪÓ∂Ø≤·œµ¡–¥∞∏