��Ŀ����
10����ش��������⣺��1������CO2Ϊֱ���η��ӵ�ԭ������̼ԭ�������Լ۲���Ӷԣ��ֱ���2����ԭ���γɦҼ������Է�������ṹΪֱ���ͻ�̼ԭ�Ӳ�ȡsp�ӻ��������ӻ�����ֱ�������������ԭ���γ�2���Ҽ������Է�������ṹΪֱ���ͣ�
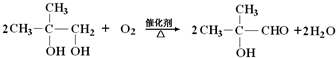
��2���״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ���Ǽ״�����ȩ���ɷ��ӹ��ɣ����Ӽ����������������ߵķе�ߵͣ��״�����֮������γ��������ȩ����֮��Ϊ���»��������¼״����Ӽ����������ڼ�ȩ���Ӽ������������Լ״��е���ڼ�ȩ��
���� ��1�������ӻ������жϣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ������Ҽ�����=��ԭ�Ӹ������µ��ӶԸ���=$\frac{1}{2}$��a-xb����aָ����ԭ�Ӽ۵��Ӹ�����xָ��ԭ�Ӹ�����bָ��ԭ���γ��ȶ��ṹ��Ҫ�ĵ��Ӹ�����CO2��Cԭ������ԭ��֮���γ�C=O������ԭ��̼��sp�������ӻ�����ֱ���̼���Ҽ���ռ�ݣ�
��2�����Ϊ���Ӽ������������Ӱ�����ʵ��۷е㣬��������ô��ڷ��»��������Ժ�������ķ��Ӿ���ķе�һ���Ըߣ��״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ������
��� �⣺��1��������̼�����м۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ���=2+$\frac{1}{2}$��4-2��2��=2������̼ԭ�Ӳ�ȡsp�ӻ���CO2��Cԭ������ԭ��֮���γ�C=O�ǣ������¶Ե��ӣ������ӻ�����ֱ�������������ԭ���γ�2���Ҽ������Է�������ṹΪֱ���ͣ�
������̼ԭ�������Լ۲���Ӷԣ��ֱ���2����ԭ���γɦҼ������Է�������ṹΪֱ���ͻ�̼ԭ�Ӳ�ȡsp�ӻ��������ӻ�����ֱ�������������ԭ���γ�2���Ҽ������Է�������ṹΪֱ���ͣ�
��2�����Ϊ���Ӽ������������Ӱ�����ʵ��۷е㣬��������ô��ڷ��»������״�����ȩ���ɷ��ӹ��ɣ��״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ�
�𣺼״�����ȩ���ɷ��ӹ��ɣ����Ӽ����������������ߵķе�ߵͣ��״�����֮������γ��������ȩ����֮��Ϊ���»��������¼״����Ӽ����������ڼ�ȩ���Ӽ������������Լ״��е���ڼ�ȩ��
���� ���⿼���˶�����̼���ӿռ乹�͵��жϡ�����������������ʵ�Ӱ�죬���ݼ۲���ӶԻ�����������������ǹؼ���ע�����Ӱ�����ʵ��۷е㣬��������ô��ڷ��»�������Ŀ�Ѷ��еȣ�

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�| A�� | �þ�����۷е�͡�Ӳ��С | |
| B�� | ������̼ԭ������C-O��ѧ����֮��Ϊ1��2 | |
| C�� | ����Ŀռ���С������6��ԭ�ӹ��� | |
| D�� | �þ�����̼ԭ�Ӻ���ԭ�ӵĸ�����Ϊ1��2 |
| A�� | ����ؿ������Ƶط�����ŵ� | |
| B�� | ������һ����Դ | |
| C�� | ͨ����ѧ��Ӧ��ʵ����������ת�� | |
| D�� | ȼ�ϵ�ص�����ת���ʿɴ�100% |
| A�� | BaCO3 | B�� | NaOH | C�� | CuSO4 | D�� | Al��OH��3 |
| A�� | ��BaCl2��Һ����SO3��g����SO2 | |
| B�� | ��Ca��OH��2��Һ����Na2CO3��Һ��NaHCO3��Һ | |
| C�� | ��CO2����Na[Al��OH��4]��Һ��Na2SiO3��Һ | |
| D�� | ��ʪ��ĵ⻯�ص�����ֽ����Br2��g����NO2 |
| A�� | ���ۺ���ά�ػ�Ϊͬ���칹�壬����ˮ��Ϊ��ԭ�Ե��� | |
| B�� | �����յķ����ɼ���ë֯�����֯�� | |
| C�� | ��������Һ�м���Ũ���������Һ�г�����������ˮ��������ܽ� | |
| D�� | ��Ȼ��֬�еľ��й̶����۷е㣬ֲ���Ͳ���ʹ������Ȼ�̼��Һ��ɫ |
| A�� | Clһ�Ľṹʾ��ͼ�� | B�� | CH4���ӵ����ģ�ͣ� | ||
| C�� | �Ȼ�þ�ĵ���ʽ�� | D�� | 8�����ӵ�̼ԭ�ӵĺ��ط��ţ�12C |
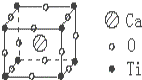 ��֪ij���������ɸơ��ѡ�������Ԫ����ɵľ��壬�侧���ṹ��ͼ��ʾ��������ʵĻ�ѧʽΪ��������
��֪ij���������ɸơ��ѡ�������Ԫ����ɵľ��壬�侧���ṹ��ͼ��ʾ��������ʵĻ�ѧʽΪ��������| A�� | CaTiO3 | B�� | CaTiO6 | C�� | Ca4TiO3 | D�� | CaTiO12 |
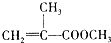 �����л������ĵ��壬������ں˴Ź�������ͼ������ʾ3����ͬ�ķ壮�����������ڼ���ϩ�����ͬ���칹����Ǣ٢ܣ���ѡ����ţ�
�����л������ĵ��壬������ں˴Ź�������ͼ������ʾ3����ͬ�ķ壮�����������ڼ���ϩ�����ͬ���칹����Ǣ٢ܣ���ѡ����ţ�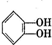 ��
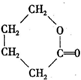
��
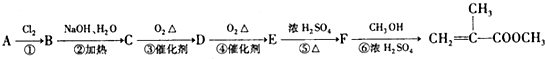
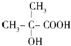 ��
�� ��
�� ��
��