��Ŀ����
11�� ����ռ������Ҫ�Ļ���ԭ�ϣ�
����ռ������Ҫ�Ļ���ԭ�ϣ���1��������ͼ��ʾװ�ÿɼ��֤��������̼���ռ���Һ�����˷�Ӧ����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ����ʱ��ʵ��������ˮ�ص����ɹ��ƿ������ƿ����ˮ����������ƿ������Ӧ�����ӷ���ʽ��2OH-+CO2=CO32-+H2O����CO2+OH-=HCO3-�����������������䣬��A��C���ӣ��ɹ۲쵽�������ǹ��ƿ�еij����ܿ������ݲ�����
��2����100mL2mol/L��NaOH ��Һ��ͨ��һ����CO2���ᾧ���õ�9.3g��ɫ���壬�ð�ɫ����������NaOH��Na2CO3��д��ѧʽ����
���ʵ��ȷ�ϸð�ɫ�����д��ڵ������ӣ���������з�����
| ʵ����� | ʵ������ | ���� |
| ��ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ | ������ɫ���� | ��CO32- |
| �ڹ��ˣ�ȡ2mL��Һ���Թ��� | ||
| �۵μӷ�̪ | ��Һ��� | ��OH- |
���� ��1����1��CO2��NaOH��Ӧ����Na2CO3����ƿ��ѹǿ��С�����жϳ��ֵ�����
��2��������Ԫ���غ��˼�룺���������е���Ԫ��ȫ��ת��Ϊ̼���ƣ�ȷ��̼����Ӧ�е��������������������Լ���Ҫ��������������ҺBaCl2�����̼���ƣ�����̼�������ȫ����������ͨ���������ڵ�pH��ȷ���Ƿ���NaOH��
��3�������������̼���������Ƶ����ʵ����������������������̼�����ʵ���֮���жϷ�Ӧ���P���ʵ�����Ȼ��д����Ӧ�����ӷ���ʽ��
��� �⣺��1����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ��������̼���ռ���Һ�����˷�Ӧ������ѹǿ��С������ˮ�ص����ɹ��ƿ������ƿ����Ӧ�����ӷ���ʽΪ2OH-+CO2=CO32-+H2O����OH-+CO2=HCO3-�����������������䣬����A��C���ӣ�������������뼯��ƿ���ɹ۲쵽�������ǹ��ƿ�еij����ܿ������ݲ�����
�ʴ�Ϊ��ˮ�ص����ɹ��ƿ������ƿ����ˮ����������ƿ����2OH-+CO2=CO32-+H2O����CO2+OH-=HCO3-�������ƿ�еij����ܿ������ݲ�����
��2��100mL2mol/L��NaOH��Һ��ͨ��һ����CO2���ᾧ���õ�NaOH��Na2CO3�Ļ����9.3g����̼���Ƶ����ʵ�����x���������Ƶ����ʵ�����y��������Ԫ���غ㣬��a��2x+y=0.2mol������������ϵ��b��40y+106x=9.3g����������a��b�����x=0.05mol��y=0.1mol��
�������Ȼ��������Ȼ��ƣ���Һ����̼���ƵĴ��ڣ�����ɫ��̪�����������ƵĴ��ڣ�����
�ʴ�Ϊ��
| ʵ����� | ʵ������ | ���� |
| ��ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ | ������ɫ���� | ��CO32- |
| �ڹ��ˣ�ȡ2mL��Һ���Թ��� | ||
| �۵μӷ�̪ | ��Һ��� | ��OH- |
�ʴ�Ϊ��2CO2+3OH-=CO32-+HCO3-+H2O��
���� ���⿼��ʵ��װ�õ��ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ����⿼���Ϊ�ۺϣ������ʵ��Ʊ�ʵ��Ϊ���壬�ۺϿ���ʵ�����ơ����ӷ���ʽ��д��֪ʶ��ע��������ʵ��������ʵ�鷽����

����ˮ�� ����ˮ�Ԣ�ǿ������ ��ǿ���ԡ��ݸ߷е㣮
| A�� | ֻ�Т٢ۢ� | B�� | ֻ�Тڢۢ� | C�� | ֻ�Тۢܢ� | D�� | �٢ڢۢܢݾ��� |
| ������� | H2CO3 | NH3•H2O |
| ����ƽ�ⳣ�� | Ka1=4.30��10-7 Ka2=5.61��10-11 | 1.77��10-5 |
������Ϊ����Һ�ʼ��ԣ���ᡱ���С��������
�ھ���Һ������֮�������й�ϵʽ������Ϊ������ȷ����ACD
A��c��NH4+����c��CO32-����c��HCO3-����c��NH3•H2O��
B��c��NH4+��+c��H+��=c��HCO3-��+c��OH-��+c��CO32-��
C��c��CO32-��+c��HCO3-��+c��H2CO3��=0.1mol•L-1
D��c��NH4+��+c��NH3•H2O��=2c��CO32-��+2c��HCO3-��+2c��H2CO3��
| A�� | Na+��Ca2+��Cl-��SO${\;}_{4}^{2-}$ | B�� | Fe2+��H+��SO${\;}_{3}^{2-}$��ClO- | ||
| C�� | K+��Fe3+��NO${\;}_{3}^{-}$��SCN- | D�� | Mg2+��NH${\;}_{4}^{+}$��Cl-��SO${\;}_{4}^{2-}$ |
| A�� | �ƾ� | B�� | ʳ�� | C�� | ���� | D�� | ����� |
| A�� | 21��5 | B�� | 11��3 | C�� | 3��1 | D�� | 4��1 |
| A�� | ����NH4HCO3 | B�� | ����Fe��OH��3 | C�� | ����NaOH | D�� | ����CaCO3 |

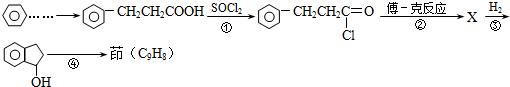
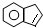 ��
��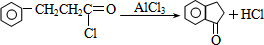 ��
��