��Ŀ����
Ϊ����̽���Ҵ������ʣ�ij��ѧ����С��ͨ���������ϲ������ʵ�鷽������̽������������ʢ��������ˮ�Ҵ����Թ��У�����һ����ȥú�͵Ľ����ƣ����Թܿ�Ѹ���������м��쵼�ܵĵ���������ȼ�ų������壬����һ�����С�ձ����ڻ����ϣ����ձ����ϳ���Һ�κ�Ѹ�ٵ�ת�ձ������ձ��м��������ij���ʯ��ˮ���۲������Dz�����
��1����д���Ҵ����Ʒ�Ӧ�Ļ�ѧ����ʽ�� ��
��2������ʵ�������ȱ�ٱ�Ҫ�IJ�������ڰ�ȫ����������ָ����ȱ�ٱ�Ҫ�IJ�����
��
��3�������ձ��м�����������ʯ��ˮ�����л��ǣ���ȼ�ղ���CO2�������������
��д���ƣ���
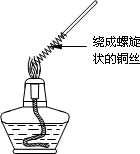
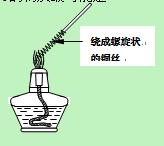
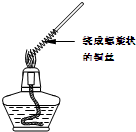
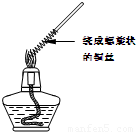
������1��ȡһ��ͭ˿��������һ���Ƴ�����״������Ӵ����������ȼһյ�ƾ��ƣ����Ƴ�����״һ�˵�ͭ˿�Ƶ��ƾ������������գ�����ͼ�����۲쵽��ʵ������ ��
��2��������״ͭ˿���ƾ��������ƶ����۲쵽��ʵ������ ���û�ѧ����ʽ��ʾ�����������ԭ���� ��
���𰸡�������������1�������û��ǻ��ϵ��⣮
��2����ȼ��ȼ������֮ǰ������鴿�ȷ�������ը��
��3���Ҵ��ӷ�����ȼ�����ɶ�����̼��
������1��ͭ˿��������Ӧ��������ͭ��
��2������ͭ���Ҵ���ԭ��ͭ���ʣ�ͬʱ�Ҵ�������Ϊ��ȩ��
����⣺������1�������û��ǻ��ϵ��⣬2Na+2CH3CH2OH=2CH3CH2ONa+H2����
��2��ȼ��ȼ������֮ǰ������鴿�ȷ�������ը���ʵ�ȼ�ų�������֮ǰû�м��鴿�ȣ�
��3����ȼ�����������������һ�ֿ��ܾ��ǻӷ������Ҵ�ȼ�����ɶ�����̼����ȼ�ղ���CO2��������������Ҵ�����������
������1��ͭ˿��������Ӧ���ɺ�ɫ������ͭ���ʹ۲쵽��ʵ������Ϊͭ˿���Ϻ�ɫ��ڣ�
��2����ɫ������ͭ���Ҵ���ԭ��ͭ��ɫ��ͭ���ʣ�ͬʱ�Ҵ�������Ϊ��ȩ����ʵ������Ϊͭ˿�ɺ�ɫ����Ϻ�ɫ����ѧ����ʽΪ��CH3CH2OH+CuO CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
�ʴ�Ϊ��������1��2Na+2CH3CH2OH=2CH3CH2ONa+H2����
��2����ȼ�ų�������֮ǰû�м��鴿�ȣ�
��3���Ҵ�����������
������1��ͭ˿���Ϻ�ɫ��ڣ�
��2��ͭ˿�ɺ�ɫ����Ϻ�ɫ��CH3CH2OH+CuO CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
���������⿼���Ҵ����������ʡ���ѧ���ʣ��ѶȲ���ע���Ҵ���������
��2����ȼ��ȼ������֮ǰ������鴿�ȷ�������ը��
��3���Ҵ��ӷ�����ȼ�����ɶ�����̼��
������1��ͭ˿��������Ӧ��������ͭ��
��2������ͭ���Ҵ���ԭ��ͭ���ʣ�ͬʱ�Ҵ�������Ϊ��ȩ��
����⣺������1�������û��ǻ��ϵ��⣬2Na+2CH3CH2OH=2CH3CH2ONa+H2����
��2��ȼ��ȼ������֮ǰ������鴿�ȷ�������ը���ʵ�ȼ�ų�������֮ǰû�м��鴿�ȣ�
��3����ȼ�����������������һ�ֿ��ܾ��ǻӷ������Ҵ�ȼ�����ɶ�����̼����ȼ�ղ���CO2��������������Ҵ�����������
������1��ͭ˿��������Ӧ���ɺ�ɫ������ͭ���ʹ۲쵽��ʵ������Ϊͭ˿���Ϻ�ɫ��ڣ�
��2����ɫ������ͭ���Ҵ���ԭ��ͭ��ɫ��ͭ���ʣ�ͬʱ�Ҵ�������Ϊ��ȩ����ʵ������Ϊͭ˿�ɺ�ɫ����Ϻ�ɫ����ѧ����ʽΪ��CH3CH2OH+CuO
 CH3CHO+Cu+H2O
CH3CHO+Cu+H2O�ʴ�Ϊ��������1��2Na+2CH3CH2OH=2CH3CH2ONa+H2����
��2����ȼ�ų�������֮ǰû�м��鴿�ȣ�
��3���Ҵ�����������
������1��ͭ˿���Ϻ�ɫ��ڣ�
��2��ͭ˿�ɺ�ɫ����Ϻ�ɫ��CH3CH2OH+CuO
 CH3CHO+Cu+H2O
CH3CHO+Cu+H2O���������⿼���Ҵ����������ʡ���ѧ���ʣ��ѶȲ���ע���Ҵ���������

��ϰ��ϵ�д�
 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ
 Ϊ����̽���Ҵ������ʣ�ij��ѧ����С��ͨ���������ϲ������ʵ�鷽������̽����
Ϊ����̽���Ҵ������ʣ�ij��ѧ����С��ͨ���������ϲ������ʵ�鷽������̽����