��Ŀ����
��1��100��ʱ��ˮKw=1×10-12 mol2?L-2�ڸ��¶��£���1mL 0.001mol?L-1��NaOH��Һ��pHΪ ������ˮϡ����100L������Һ��pHΪ ����2�������£�pH=1��ϡ����a L��pH=12�Ŀ�����b L��Ϻ���Һ��pH=2��������Һ���ǰ������仯����a��b= ��
��3����֪25��ʱ100gˮ������0.74g��Ca��OH��2���ﵽ���ͣ����������Ca��OH��2���ӻ�ΪKsp= ��
���𰸡���������1���ȼ���c��OH-��������Kw=c��H+��?c��OH-��=1×10-14��������Һ��c��H+����������pH=-lgc��H+�����㣻
��2������кͷ�Ӧ���ȼ������Ϻ���Һ�е�������Ũ�ȣ�������pH=-lgc��H+���г���ʽ���м��㣻
��3���ȸ����������Ƶ��ܽ�����������Һ�еĸ�����Ũ�Ⱥ�����������Ũ�ȣ��ٸ��ݣ�Ca2+��×c��OH-��2��⣻
����⣺��1��1mL 0.001mol?L-1��NaOH��Һ��c��OH-��=c��NaOH��=1×10-3mol/L������c��H+��= �T1×10-9mol/L�����Ը���Һ��pH=-lg��1×10-9��=9����1mL 0.001mol?L-1��NaOH��Һ����ˮϡ����100L��NaOH��Һ��c��OH-����1×10-6mol/L�����Ը���Һ��pH=-lg��1×10-6��=6���Գʼ��ԣ��ӽ����ԣ�
�T1×10-9mol/L�����Ը���Һ��pH=-lg��1×10-9��=9����1mL 0.001mol?L-1��NaOH��Һ����ˮϡ����100L��NaOH��Һ��c��OH-����1×10-6mol/L�����Ը���Һ��pH=-lg��1×10-6��=6���Գʼ��ԣ��ӽ����ԣ�
�ʴ�Ϊ��9�� 6��
��2�������£�pH=1��ϡ����c��H+��=0.1mol/L��pH=12�Ŀ�����c��OH-��=c��NaOH��=0.01mol/L��Ҫʹ�û�Ϻ���ҺpH=2�����Ϻ� =0.01mol/L����
=0.01mol/L����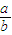 =2��9��
=2��9��
�ʴ�Ϊ��2��9��
��3��25��ʱ100gˮ������0.74g��Ca��OH��2���ﵽ���ͣ����������100��ˮ���ܽ�0.74��Ca��OH��2�����ʵ���Ϊ0.01mol����ʱ��Һ������100.74�ˣ���Һ�����100.74g÷1g/cm3=100.74mL����ʱCa��OH��2���ʵ���Ũ��Ϊ��C= =
= =0.099mol/L�����ԣ���ʱCa2+���ʵ���Ũ��Ϊ��0.099mol/L��OH-���ʵ���Ũ��Ϊ��0.198mol/L��Ksp=c��Ca2+��×c��OH-��2=0.004 mol3?L-3��
=0.099mol/L�����ԣ���ʱCa2+���ʵ���Ũ��Ϊ��0.099mol/L��OH-���ʵ���Ũ��Ϊ��0.198mol/L��Ksp=c��Ca2+��×c��OH-��2=0.004 mol3?L-3��
�ʴ�Ϊ��0.004 mol3?L-3��
������������Ҫ��������Һ�е�PH���㣬������������ü��㹫ʽ����Ŀ�Ѷ��еȣ�
��2������кͷ�Ӧ���ȼ������Ϻ���Һ�е�������Ũ�ȣ�������pH=-lgc��H+���г���ʽ���м��㣻
��3���ȸ����������Ƶ��ܽ�����������Һ�еĸ�����Ũ�Ⱥ�����������Ũ�ȣ��ٸ��ݣ�Ca2+��×c��OH-��2��⣻
����⣺��1��1mL 0.001mol?L-1��NaOH��Һ��c��OH-��=c��NaOH��=1×10-3mol/L������c��H+��=
 �T1×10-9mol/L�����Ը���Һ��pH=-lg��1×10-9��=9����1mL 0.001mol?L-1��NaOH��Һ����ˮϡ����100L��NaOH��Һ��c��OH-����1×10-6mol/L�����Ը���Һ��pH=-lg��1×10-6��=6���Գʼ��ԣ��ӽ����ԣ�
�T1×10-9mol/L�����Ը���Һ��pH=-lg��1×10-9��=9����1mL 0.001mol?L-1��NaOH��Һ����ˮϡ����100L��NaOH��Һ��c��OH-����1×10-6mol/L�����Ը���Һ��pH=-lg��1×10-6��=6���Գʼ��ԣ��ӽ����ԣ��ʴ�Ϊ��9�� 6��
��2�������£�pH=1��ϡ����c��H+��=0.1mol/L��pH=12�Ŀ�����c��OH-��=c��NaOH��=0.01mol/L��Ҫʹ�û�Ϻ���ҺpH=2�����Ϻ�
 =0.01mol/L����
=0.01mol/L����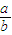 =2��9��
=2��9���ʴ�Ϊ��2��9��
��3��25��ʱ100gˮ������0.74g��Ca��OH��2���ﵽ���ͣ����������100��ˮ���ܽ�0.74��Ca��OH��2�����ʵ���Ϊ0.01mol����ʱ��Һ������100.74�ˣ���Һ�����100.74g÷1g/cm3=100.74mL����ʱCa��OH��2���ʵ���Ũ��Ϊ��C=
 =
= =0.099mol/L�����ԣ���ʱCa2+���ʵ���Ũ��Ϊ��0.099mol/L��OH-���ʵ���Ũ��Ϊ��0.198mol/L��Ksp=c��Ca2+��×c��OH-��2=0.004 mol3?L-3��
=0.099mol/L�����ԣ���ʱCa2+���ʵ���Ũ��Ϊ��0.099mol/L��OH-���ʵ���Ũ��Ϊ��0.198mol/L��Ksp=c��Ca2+��×c��OH-��2=0.004 mol3?L-3���ʴ�Ϊ��0.004 mol3?L-3��
������������Ҫ��������Һ�е�PH���㣬������������ü��㹫ʽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
������ʵ����֤��ij��ɫ��Һ���Ǵ�ˮ���ǣ�������
| A����1.01��105Paʱ��ø�Һ��ķе���100�� | B�����³�ѹ�£���ø�Һ���pH=7 | C������Һ��ʱ�õ�H2��O2�������Ϊ2��1����ͬ������ | D��һС��������ܸ��ڸ�Һ�棬Ѹ���ζ����������壬����˻˻�� |