��Ŀ����
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�ꡣ
��1��250��ʱ�������Ͻ�Ϊ��������4 L������ͨ��6 mol CO2��6 mol CH4���������·�Ӧ��CO2(g)��CH4(g) 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
| ���� | CH4 | CO2 | CO | H2 |
| ������� | 0.1 | 0.1 | 0.4 | 0.4 |
�ٴ��¶��¸÷�Ӧ��ƽ�ⳣ��K= ��
����֪��CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H="-890.3" kJ��mol��1
CO(g)��H2O (g)��CO2(g)��H2 (g) ��H="2.8" kJ��mol��1
2CO(g)��O2(g)��2CO2(g) ��H="-566.0" kJ��mol��1
��ӦCO2(g)��CH4(g)
 2CO(g)��2H2(g) �ġ�H= ��
2CO(g)��2H2(g) �ġ�H= ����2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᡣ
���ڲ�ͬ�¶��´����Ĵ�Ч�������������������ͼ��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ���� ��
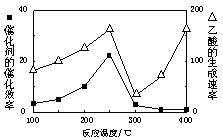
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
�۽�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ ��
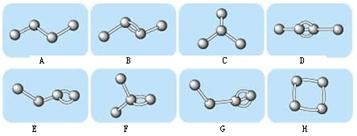
��3����CO2Ϊԭ�Ͽ��Ժϳɶ������ʡ�
�پ�̼������һ����������ͺϳɲ��ϣ������ɼӾ۶��ɡ�д����̼�����Ľṹ��ʽ�� ��
������������ˮ��Һ������ʽ��е�⣬CO2��ͭ�缫�Ͽ�ת��Ϊ���飬�õ缫��Ӧ����ʽΪ ��
��1����64 ��+247.3 kJ��mol��1
��2�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ���
������Ӧѹǿ������CO2��Ũ��
��3Cu2Al2O4+32H++2NO3��=6Cu2++ 6Al3++2NO��+16 H2O
��3����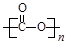 ��CO2+8e��+6H2O=CH4+8OH��
��CO2+8e��+6H2O=CH4+8OH��
���������������1����������CH4���ʵ���Ϊx����ƽ��ʱCH4��CO2��CO��H2���ʵ����ֱ�Ϊ(6-x)mol��(6-x)mol��2xmol��2xmol����CH4���������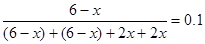 �����x=4������K=
�����x=4������K= 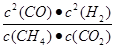 =
= 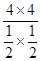 =64�����ɸ�˹���ɵá�H=(-890.3+2.8��2+566��2) kJ��mol��1=+247.3kJ��mol��1����2�����¶����ߣ��������Խ��͡���CO2(g)��CH4(g)
=64�����ɸ�˹���ɵá�H=(-890.3+2.8��2+566��2) kJ��mol��1=+247.3kJ��mol��1����2�����¶����ߣ��������Խ��͡���CO2(g)��CH4(g) CH3COOH(l)������ѹǿ�����������̼����Ũ�ȣ����������ת���ʡ���Cu2Al2O4��CuΪ+1�ۣ�Cu2Al2O4��������������ͭ����������NO��ˮ�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ����3����CO2�����Ӿ۷�Ӧʱ������C=O˫���е�
CH3COOH(l)������ѹǿ�����������̼����Ũ�ȣ����������ת���ʡ���Cu2Al2O4��CuΪ+1�ۣ�Cu2Al2O4��������������ͭ����������NO��ˮ�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ����3����CO2�����Ӿ۷�Ӧʱ������C=O˫���е�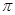 �����ۺϵ�
�����ۺϵ�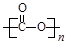 ����CO2��ͭ�缫�Ͽ�ת��Ϊ���飬C�Ļ��ϼ���+4��Ϊ-4��������ԭ��Ӧ���ɵ��Ӻ͵���غ㡢�����غ�д���缫��Ӧʽ��
����CO2��ͭ�缫�Ͽ�ת��Ϊ���飬C�Ļ��ϼ���+4��Ϊ-4��������ԭ��Ӧ���ɵ��Ӻ͵���غ㡢�����غ�д���缫��Ӧʽ��
���㣺 ��ѧƽ�ⳣ�� ��˹���� ��ѧƽ���ƶ���ת���� ���ӷ���ʽ �߾��� �缫��Ӧʽ����д

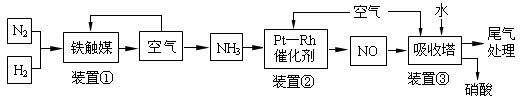
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ� ũҵ������������������Ҫ���ã� 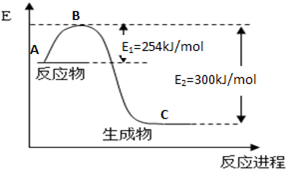
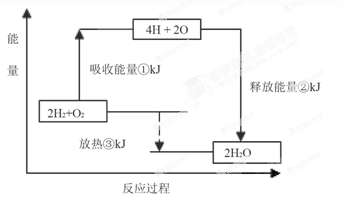
��1����ͼ��N2(g)��H2(g)��Ӧ����1mol NH3(g)�����������仯ʾ��ͼ����д��N2��H2��Ӧ���Ȼ�ѧ����ʽ�� ��
��2������֪�������ݣ�
| ��ѧ�� | H��H | N��N |
| ����/kJ��mol��1 | 435 | 943 |
�Ը��ݱ��м�ͼ�����ݼ���N-H�ļ��� kJ��mol��1��
��3���ϳɰ���Ӧͨ��������ý��������ʹ������ý��E1��E2�ı仯�ǣ�E1 ��E2______��
��H (���������С���������䡱)��
��4����NH3����ԭNOX���������������������Ⱦ������
4NH3(g)+3O2(g)�� 2N2(g)+6H2O(g) ����H1��akJ��mol-1
N2(g)+O2(g)��2NO(g)�� ��H2��bkJ/mol
��1mol NH3��ԭNO��N2����÷�Ӧ�����еķ�Ӧ�ȡ�H3�� kJ/mol���ú�a��b��ʽ�ӱ�ʾ����
��������;�㷺����¯�������ܷ�ӦΪ��Fe2O3��s��+3CO��g�� 2Fe��s��+3CO2��g������ش��������⣺
2Fe��s��+3CO2��g������ش��������⣺
��1��һ���¶��£�������̶����ܱ������з���������Ӧ�������жϸ÷�Ӧ�Ѿ��ﵽƽ����� ��
| A���ܱ���������ѹǿ���� |
| B���ܱ������л�������ƽ��Ħ���������� |
| C���ܱ������л��������ܶȲ��� |
| D��c��CO��= c��CO2�� |
��2��һ���¶��£�������Ӧ�Ļ�ѧƽ�ⳣ��Ϊ3.0�����¶��½�4molCO��2molFe2O3��6molCO2��5molFe�����ݻ�Ϊ2L���ܱ������У���ʱ��Ӧ���� ��Ӧ������У���������桱����ƽ��״̬��������Ӧ��ƽ����������¶ȣ�CO��CO2�����������������ӦΪ ��Ӧ������ȡ����ȡ��� ��
��3����֪��3Fe2O3��s��+CO��g��
 2Fe3O4��s��+CO2��g�� ��H="�C47" kJ/mol
2Fe3O4��s��+CO2��g�� ��H="�C47" kJ/molFe3O4��s��+CO��g��
 3FeO��s��+CO2��g�� ��H=" +19" kJ/mol
3FeO��s��+CO2��g�� ��H=" +19" kJ/molFeO��s��+CO��g��
 Fe��s��+CO2��g�� ��H="�C11" kJ/mol
Fe��s��+CO2��g�� ��H="�C11" kJ/mol��Fe2O3��s��+3CO��g��
 2Fe��s��+3CO2��g���ġ�H= ��
2Fe��s��+3CO2��g���ġ�H= ����4�������ܷ�Ӧ�ڸ�¯�д��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���
| �¶� | 250�� �� 600�� �� 1000�� �� 2000�� |
| ��Ҫ�ɷ� | Fe2O3 Fe3O4 FeO Fe |
800��ʱ�������ʵ���Ҫ�ɷ�Ϊ �����¶�������ù���������m(Fe)�Um(O)=105�U32����Fe3O4��CO��ԭΪFeO�İٷ���Ϊ �����������������в���Fe��OԪ�أ���
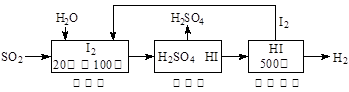
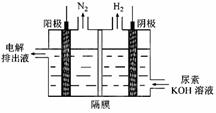
 Ni(OH)2��M
Ni(OH)2��M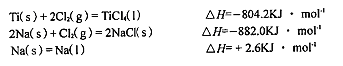
 2NH3(g) ��H����92.4 kJ��mol-1��Ӱ�졣ʵ������ͼ��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����
2NH3(g) ��H����92.4 kJ��mol-1��Ӱ�졣ʵ������ͼ��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����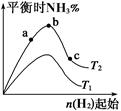
 2NO(g) ��H����180.5 kJ��mol-1
2NO(g) ��H����180.5 kJ��mol-1
 N2 + H2O + nX��δ��ƽ�ķ�Ӧʽ����
N2 + H2O + nX��δ��ƽ�ķ�Ӧʽ����