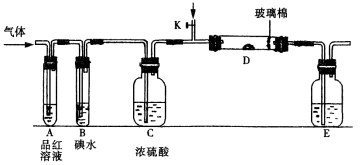
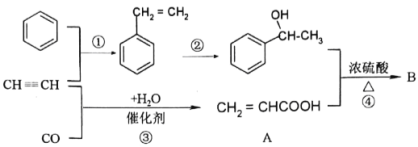
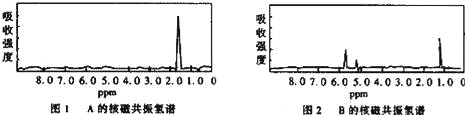
��Ŀ����
����Ŀ������ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2(g)��O2(g)===2H2O(1)����H����572 kJ��mol��1����ش��������⣺
(1)�����������ܺ�________(����ڡ���С�ڡ����ڡ�)��Ӧ�������ܺ͡�
(2)��2 mol����ȼ������ˮ��������ų�������________(����ڡ���С�ڡ����ڡ�)572 kJ��
(3)H2�ı�ȼ���Ȧ�H��________��
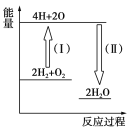
(4)��Ӧ2H2��O2![]() 2H2O�������仯��ͼ��ʾ��
2H2O�������仯��ͼ��ʾ��
��֪��1 mol H2��1 molO2��1 mol H��O�еĻ�ѧ���ֱ���Ҫ����436 kJ��496 kJ��463 kJ��������
��Ӧ����(��)________(����ա��ų���)________kJ��
���𰸡�(1)С�ڡ�(2)С�ڡ�(3)��286 kJ��mol��1��(4)�ų� 1852
��������(1)�÷�Ӧ�Ħ�H<0��Ϊ���ȷ�Ӧ�������������ܺ�С�ڷ�Ӧ�������ܺ͡�
(2)���Ȼ�ѧ����ʽ��֪��2 mol����ȼ������Һ̬ˮ���ų�572 kJ����������Һ̬ˮ��Ϊˮ������Ҫ������������2 mol����ȼ������ˮ�������ų�������С��572 kJ��
(3)��ȼ���Ȧ�H����![]() kJ��mol��1������286 kJ��mol��1��
kJ��mol��1������286 kJ��mol��1��
(4)��Ӧ����(1)�У������ɵ͵��ߣ���Ӧ������������Ӧ����(��)�У������ɸߵ��ͣ���Ӧ�ų���������Ӧ����(��)���γ���4 mol H��O�����ų�������Ϊ��4��463 kJ��1 852 kJ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������������������;�Ķ�Ӧ��ϵ����ȷ���ǣ� ��
ѡ�� | ���� | ��; |
A | �������ʺ���ɫ | ��ɫͿ�� |
B | �������۵�ܸ� | �ͻ���� |
C | Ũ���������ˮ�� | ����� |
D | Һ������ʱҪ���մ������� | ����� |
A��A B��B C��C D��D