��Ŀ����
����Ŀ����(Sb)���仯�����ڹ�ҵ����������;���Ի������Ҫ�ɷ�ΪSb2S3�������� PbS��As2S3��CuO��SiO2�ȣ�Ϊԭ���Ʊ�������Ĺ���������ͼ��ʾ��
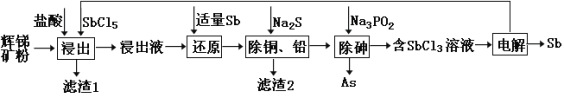
��֪���� ����Һ�г������������SbCl5֮�⣬������SbCl3��PbCl2��AsCl3��CuCl2�ȣ�
�ڳ����£�Ksp(CuS)=1.27��10-36��Ksp(PbS)=9.04��10-29��
����Һ������Ũ��С�ڻ����1.0��10-5mol/Lʱ����Ϊ�����ӳ�����ȫ��
(1)����1�г���S֮�⣬����___________���ѧʽ����
(2)��������ʱ��Sb2S3������Ӧ�Ļ�ѧ����ʽΪ_________��
(3)������������ʵ�֣��ѧʽ����ѭ������ __________��
(4)�����£�����ͭ��Ǧ��ʱ��Cu2+��Pb2+��������ȫ����ʱ��Һ�е�c(S2-)������______��
����Na2SҲ���˹��࣬��ԭ��Ϊ______________��
(5)��������ʱ��H3PO3���ɣ��÷�Ӧ�����ӷ���ʽΪ__________��
(6)�������ʱ����������SbԪ���뱻��ԭ��SbԪ�ص�����֮��Ϊ_______��
���𰸡�SiO2 Sb2S3��3SbCl5=5SbCl3��3S SbCl5��Sb 9.04��10-24mol/L ����H2S����Ⱦ�����������Sb2S3 2As3+��3PO23-��3H+��3H2O=2As����3H3PO3 3:2
��������
�����(��Ҫ�ɷ�ΪSb2S3��������PbS��As2S3��CuO��SiO2��)���������ȡ�õ���ȡҺ������Һ�г������������SbCl5֮�⣬������SbCl3��PbCl2��AsCl3��CuCl2�ȣ�����1�г������ɵ�S֮���Ϊ�ܽ�Ķ������裬����Һ�м���Sb��ԭSbCl5������SbCl3������Na2Sʱ��֤Cu2+��Pb2+��������ȫ�����˵õ�����2ΪCuS��PbS����Һ�м���Na3PO2��Һ���飬������������鵥�ʣ�ʣ��SbCl3��Һ��ͨ����õ�Sb���ݴ˷������
(1)������֪����1�г���S֮�⣬����SiO2��
(2)��������ʱ֮��Sb2S3��HCl��Һ�к�SbCl5����������ԭ��Ӧ�����ɵ������+3�۵��Ȼ������ԭ���غ㼰�����غ㣬�ɵ÷�Ӧ�Ļ�ѧ����ʽΪ��Sb2S3+3SbCl5=5SbCl3+3S��
(3)��������ͼ��֪Sb��ԭSbCl5ΪSbCl3�������SbCl3��Һ�õ�Sb���ʼ�SbCl5��SbCl5�����ڿ�ʼʱ�ܽ�����ۣ����Կ���ѭ�����õ�������SbCl5��Sb��
(4)�����£�Ksp(CuS)=1.27��10-36��Ksp(PbS)=9.04��10-29�������£�����ͭ��Ǧ��ʱ��Cu2+��Pb2+��������ȫ���ܶȻ�������֪����Ǧȫ������ʱ��ͭ������ȫ����Һ������Ũ��С�ڵ���1.0��10-5mol/Lʱ����Ϊ�����ӳ�����ȫ����ʱ��Һ�е�c(S2-)��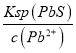 =
=![]() =9.04��10-24 mol/L��Na2SҲ���˹��࣬��������H2S����Ⱦ�����������Sb2S3��
=9.04��10-24 mol/L��Na2SҲ���˹��࣬��������H2S����Ⱦ�����������Sb2S3��
(5)��Һ�м���Na3PO2��Һ���飬������������鵥�ʣ���Ӧ�Ļ�ѧ����ʽΪ��2AsCl3+3Na3PO2+3HCl+3H2O=2As+3H3PO3+9NaCl����Ӧ�����ӷ���ʽΪ2As3++3PO23-+3H++3H2O=2As��+3H3PO3��
(6)��������ͼ��֪���ڵ��ʱSbCl3��Ӧ����SbCl5��Sb�������غ���㣬Sb3+��Sb5+��2e-��Sb3+��������Sb3+��Sb��3e-��Sb3+����ԭ������������ԭ��Ӧ�е����غ��֪��������SbԪ���뱻��ԭ��SbԪ�����ʵ���֮��Ϊ3��2��